How geographic access to care shapes disease burden: The current impact of post-exposure prophylaxis and potential for expanded access to prevent human rabies deaths in Madagascar
- PMID: 33901194
- PMCID: PMC8102000
- DOI: 10.1371/journal.pntd.0008821
How geographic access to care shapes disease burden: The current impact of post-exposure prophylaxis and potential for expanded access to prevent human rabies deaths in Madagascar
Abstract
Background: Post-exposure prophylaxis (PEP) is highly effective at preventing human rabies deaths, however access to PEP is limited in many rabies endemic countries. The 2018 decision by Gavi to add human rabies vaccine to its investment portfolio should expand PEP availability and reduce rabies deaths. We explore how geographic access to PEP impacts the rabies burden in Madagascar and the potential benefits of improved provisioning.
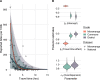
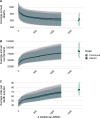
Methodology & principal findings: We use spatially resolved data on numbers of bite patients seeking PEP across Madagascar and estimates of travel times to the closest clinic providing PEP (N = 31) in a Bayesian regression framework to estimate how geographic access predicts reported bite incidence. We find that travel times strongly predict reported bite incidence across the country. Using resulting estimates in an adapted decision tree, we extrapolate rabies deaths and reporting and find that geographic access to PEP shapes burden sub-nationally. We estimate 960 human rabies deaths annually (95% Prediction Intervals (PI): 790-1120), with PEP averting an additional 800 deaths (95% PI: 640-970) each year. Under these assumptions, we find that expanding PEP to one clinic per district (83 additional clinics) could reduce deaths by 19%, but even with all major primary clinics provisioning PEP (1733 additional clinics), we still expect substantial rabies mortality. Our quantitative estimates are most sensitive to assumptions of underlying rabies exposure incidence, but qualitative patterns of the impacts of travel times and expanded PEP access are robust.
Conclusions & significance: PEP is effective at preventing rabies deaths, and in the absence of strong surveillance, targeting underserved populations may be the most equitable way to provision PEP. Given the potential for countries to use Gavi funding to expand access to PEP in the coming years, this framework could be used as a first step to guide expansion and improve targeting of interventions in similar endemic settings where PEP access is geographically restricted and baseline data on rabies risk is lacking. While better PEP access should save many lives, improved outreach, surveillance, and dog vaccination will be necessary, and if rolled out with Gavi investment, could catalyze progress towards achieving zero rabies deaths.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- World Health Organization. World health statistics 2018: Monitoring health for the SDGs Sustainable Development Goals. World Health Organization; 2018.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

