Beyond dissolution: Xerostomia rinses affect composition and structure of biomimetic dental mineral in vitro
- PMID: 33901259
- PMCID: PMC8075190
- DOI: 10.1371/journal.pone.0250822
Beyond dissolution: Xerostomia rinses affect composition and structure of biomimetic dental mineral in vitro
Abstract
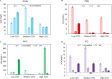
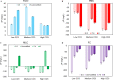
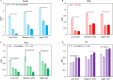
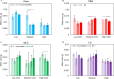
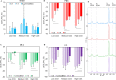
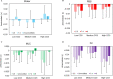
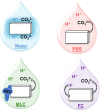
Xerostomia, known as dry mouth, is caused by decreased salivary flow. Treatment with lubricating oral rinses provides temporary relief of dry mouth discomfort; however, it remains unclear how their composition affects mineralized dental tissues. Therefore, the objective of this study was to analyze the effects of common components in xerostomia oral rinses on biomimetic apatite with varying carbonate contents. Carbonated apatite was synthesized and exposed to one of the following solutions for 72 hours at varying pHs: water-based, phosphorus-containing (PBS), mucin-like containing (MLC), or fluoride-containing (FC) solutions. Post-exposure results indicated that apatite mass decreased irrespective of pH and solution composition, while solution buffering was pH dependent. Raman and X-ray diffraction analysis showed that the addition of phosphorus, mucin-like molecules, and fluoride in solution decreases mineral carbonate levels and changed the lattice spacing and crystallinity of bioapatite, indicative of dissolution/recrystallization processes. The mineral recrystallized into a less-carbonated apatite in the PBS and MLC solutions, and into fluorapatite in FC. Tap water did not affect the apatite lattice structure suggesting formation of a labile carbonate surface layer on apatite. These results reveal that solution composition can have varied and complex effects on dental mineral beyond dissolution, which can have long term consequences on mineral solubility and mechanics. Therefore, clinicians should consider these factors when advising treatments for xerostomia patients.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Bartels C. Xerostomia. Available from: https://oralcancerfoundation.org/complications/xerostomia/, 2019.
-
- Sreebny LM, Vissink A. Dry Mouth: The Malevolent Symptom: A Clinical Guide. Ames, Iowa, Ames, Iowa. Wiley-Blackwell, 2010.
-
- Department of Scientific Information, ADA Science Institute. Xerostomia (Dry Mouth). Available from: https://www.ada.org/en/member-center/oral-health-topics/xerostomia, 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

