Performance evaluation of the QIAstat-Dx® Respiratory SARS-CoV-2 Panel
- PMID: 33901651
- PMCID: PMC8064816
- DOI: 10.1016/j.ijid.2021.04.066
Performance evaluation of the QIAstat-Dx® Respiratory SARS-CoV-2 Panel
Abstract
Objective: The aim of this study was to evaluate the QIAstat-Dx® Respiratory SARS-CoV-2 Panel (QIAstat-SARS-CoV-2), which is a closed, fully automated, multiplex polymerase chain reaction (PCR) assay that detects severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and 21 other pathogens that cause respiratory disease.
Methods: Nasopharyngeal swabs from patients with or suspected of having coronavirus disease 2019 were collected and tested at Bichat-Claude Bernard Hospital, Paris, France. Using the World Health Organisation-approved real-time-PCR assay developed by the Charité Institute of Virology as the reference, positive percent agreement (PPA) and negative percent agreement (NPA) were calculated.
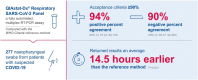
Results: In total, 189 negative and 88 positive samples were analyzed. QIAstat-SARS-CoV-2 had an NPA of 90.48% (95% confidence interval (CI), 85.37%, 94.26%) and a PPA of 94.32% (95% CI, 87.24%, 98.13%). Co-infections were detected by QIAstat-SARS-CoV-2 in 4/277 specimens. The methods exhibited comparable failure rates (23/307 [7.5%] vs. 6/298 [2.0%] for QIAstat-SARS-CoV-2 and reference methods, respectively). The turnaround time was shorter for QIAstat-SARS-CoV-2 compared with the reference method (difference in mean -14:30 h [standard error, 0:03:23; 95% CI, -14:37, -14:24]; P < 0.001).
Conclusions: QIAstat-SARS-CoV-2 shows good agreement with the reference assay, providing faster and accurate results for detecting SARS-CoV-2.
Keywords: COVID-19; Diagnostic testing; Multiplex; Real time-PCR; SARS-CoV-2.
Copyright © 2021 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures
References
-
- Brendish N., Poole S., Naidu V., Mansbridge C., Norton N., Wheeler H., et al. Clinical impact of molecular point-of-care testing for suspected COVID-19 in hospital (COV-19POC): a prospective, interventional, non-randomized, controlled study. Lancet Respir Med. 2020;8(December (12)):1192–1200. doi: 10.1016/S2213-2600(20)30454-9. - DOI - PMC - PubMed
-
- European Centre for Disease Prevention and Control . 2020. Diagnostic testing and screening for SARS-CoV-2. Available from: https://www.ecdc.europa.eu/en/covid-19/latest-evidence/diagnostic-testing. [Accessed October 2020]
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous