The retinal pigment epithelium: Development, injury responses, and regenerative potential in mammalian and non-mammalian systems
- PMID: 33901682
- PMCID: PMC8536801
- DOI: 10.1016/j.preteyeres.2021.100969
The retinal pigment epithelium: Development, injury responses, and regenerative potential in mammalian and non-mammalian systems
Abstract
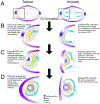
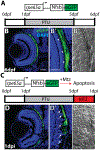
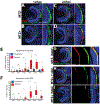
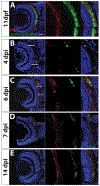
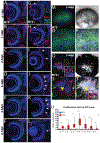
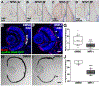
Diseases that result in retinal pigment epithelium (RPE) degeneration, such as age-related macular degeneration (AMD), are among the leading causes of blindness worldwide. Atrophic (dry) AMD is the most prevalent form of AMD and there are currently no effective therapies to prevent RPE cell death or restore RPE cells lost from AMD. An intriguing approach to treat AMD and other RPE degenerative diseases is to develop therapies focused on stimulating endogenous RPE regeneration. For this to become feasible, a deeper understanding of the mechanisms underlying RPE development, injury responses and regenerative potential is needed. In mammals, RPE regeneration is extremely limited; small lesions can be repaired by the expansion of adjacent RPE cells, but large lesions cannot be repaired as remaining RPE cells are unable to functionally replace lost RPE tissue. In some injury paradigms, RPE cells proliferate but do not regenerate a morphologically normal monolayer, while in others, proliferation is pathogenic and results in further disruption to the retina. This is in contrast to non-mammalian vertebrates, which possess tremendous RPE regenerative potential. Here, we discuss what is known about RPE formation during development in mammalian and non-mammalian vertebrates, we detail the processes by which RPE cells respond to injury, and we describe examples of RPE-to-retina and RPE-to-RPE regeneration in non-mammalian vertebrates. Finally, we outline barriers to RPE-dependent regeneration in mammals that could potentially be overcome to stimulate a regenerative response from the RPE.
Keywords: Age-related macular degeneration (AMD); Development; Regeneration; Retinal pigment epithelium (RPE); Zebrafish.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
L.L.L. is co-inventor on a US Patent (#9,458,428) related to deriving RPE from pluripotent stem cells
Figures

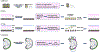
References
-
- Al-Hussaini H, Kilarkaje N, Shahabi G, Al-Mulla F, 2016. Proliferation and Migration of Peripheral Retinal Pigment Epithelial Cells Are Associated with the Upregulation of Wingless-Related Integration and Bone Morphogenetic Protein Signaling in Dark Agouti Rats. Med Princ Pract 25, 408–416. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

