Small-molecule inhibition of METTL3 as a strategy against myeloid leukaemia
- PMID: 33902106
- PMCID: PMC7613134
- DOI: 10.1038/s41586-021-03536-w
Small-molecule inhibition of METTL3 as a strategy against myeloid leukaemia
Abstract
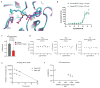
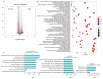
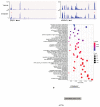
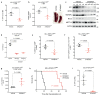
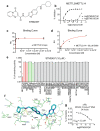
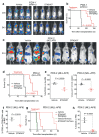
N6-methyladenosine (m6A) is an abundant internal RNA modification1,2 that is catalysed predominantly by the METTL3-METTL14 methyltransferase complex3,4. The m6A methyltransferase METTL3 has been linked to the initiation and maintenance of acute myeloid leukaemia (AML), but the potential of therapeutic applications targeting this enzyme remains unknown5-7. Here we present the identification and characterization of STM2457, a highly potent and selective first-in-class catalytic inhibitor of METTL3, and a crystal structure of STM2457 in complex with METTL3-METTL14. Treatment of tumours with STM2457 leads to reduced AML growth and an increase in differentiation and apoptosis. These cellular effects are accompanied by selective reduction of m6A levels on known leukaemogenic mRNAs and a decrease in their expression consistent with a translational defect. We demonstrate that pharmacological inhibition of METTL3 in vivo leads to impaired engraftment and prolonged survival in various mouse models of AML, specifically targeting key stem cell subpopulations of AML. Collectively, these results reveal the inhibition of METTL3 as a potential therapeutic strategy against AML, and provide proof of concept that the targeting of RNA-modifying enzymes represents a promising avenue for anticancer therapy.
Conflict of interest statement
T.K. is a co-founder of Abcam Plc and Storm Therapeutics Ltd, Cambridge, UK and Scientific Advisor to Foghorn Therapeutics and EpiVario. E.Y. is funded by Storm Therapeutics Ltd, Cambridge, UK. W.B., M.A., G.T., D.L., B.A., R.F., A.G.H., N.A.W., P.G. and O.R. are employees of Storm Therapeutics Ltd, Cambridge, UK. E.S.P. is an employee of Evotec (UK) Ltd, Abingdon, UK. George S. Vassiliou is a consultant for Kymab, Cambridge, UK. Storm Therapeutics Ltd is the owner of a patent application (WO2020201773) covering the development of METTL3 RNA methyltrasferase inhibitors.
Figures






Comment in
-
METTLing with RNA methylation in leukaemia.Nat Rev Drug Discov. 2021 Jun;20(6):423. doi: 10.1038/d41573-021-00071-1. Nat Rev Drug Discov. 2021. PMID: 33907314 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

