Investigation of Physiological Effects Induced by Dehydroepiandrosterone in Human Endothelial Cells and Ovarian Cancer Cell Line
- PMID: 33902257
- PMCID: PMC8100783
- DOI: 10.4274/tjps.galenos.2020.58827
Investigation of Physiological Effects Induced by Dehydroepiandrosterone in Human Endothelial Cells and Ovarian Cancer Cell Line
Abstract
Objectives: Dehydroepiandrosterone (DHEA) is an endogenous hormone that acts as a ligand for several cellular receptors. An age-dependent decline in circulating levels of DHEA is linked to changes in various physiological functions. In gynecological clinical practice, DHEA is commonly prescribed to induce ovulation. Some clinical studies report a positive association between high serum concentrations of DHEA and an increased risk of developing ovarian cancer. However, the in vitro physiological effects of DHEA on ovarian cancerous cells have not been explored thus far. In this study, we aimed to investigate the physiological effects of DHEA treatment (0-200 μM, 24-72 hours) on MDAH-2774 human ovarian cancer cell line and primary HuVeC human endothelial cells.
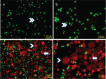
Materials and methods: The physiological effects of DHEA treatment (0-200 μM, 24-72 hours) on MDAH-2774 human ovarian cancer cell line and primary HuVeC human endothelial cells were investigated with the (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) test, acridine orange/ethidium bromide staining, and scratch assay.
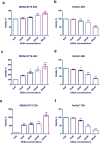
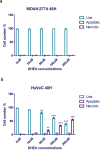
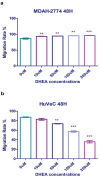
Results: DHEA treatment promoted proliferation of the MDAH-2774 cancer cell line in a dose-dependent manner (r=0.6906, p<0.0001, for 24 hours) (r=0.6802, p<0.0001, for 48 hours) (r=0.7969, p<0.0001, for 72 hours). In contrast, DHEA inhibited proliferation of the primary HuVeC cells (r=0.9490, p<0.0001, for 24 hours) (r=0.9533, p<0.0001, for 48 hours) (r=0.9584, p<0.0001, for 72 hours). In agreement with these observations, DHEA treatment resulted in a dose-dependent increase in the number of necrotic cells in the primary HuVeC cells (r=0.97, p<0.0001). However, the number of necrotic or apoptotic cells did not change significantly when the MDAH-2774 cells was exposed to DHEA. Moreover, we found that DHEA treatment reduced the migration rate of HuVeC cells in a dose-dependent manner (r=0.9868, p<0.0001), whereas only a slight increase was observed in the MDAH-2774 ovarian cancer cell line (r=0.8938, p<0.05).
Conclusion: Our findings suggest that DHEA promotes the proliferation of ovarian cancer cells in a dose-dependent manner in vitro. Moreover, DHEA induced necrosis and inhibited proliferation in endothelial cells. Although mechanistic evidence is required, our preliminary findings imply that exposure to high doses of DHEA may be associated with an increased risk of developing ovarian cancer.
Keywords: Dehydroepiandrosterone; HuVeC; MDAH-2774; ovarian cancer.
Conflict of interest statement
Figures

Similar articles
-
In Vitro Physiological Effects of Betahistine on Cell Lines of Various Origins.Turk J Pharm Sci. 2021 Apr 20;18(2):140-145. doi: 10.4274/tjps.galenos.2020.88155. Turk J Pharm Sci. 2021. PMID: 33900698 Free PMC article.
-
Dehydroepiandrosterone inhibits the proliferation and induces the death of HPV-positive and HPV-negative cervical cancer cells through an androgen- and estrogen-receptor independent mechanism.FEBS J. 2009 Oct;276(19):5598-609. doi: 10.1111/j.1742-4658.2009.07253.x. Epub 2009 Aug 21. FEBS J. 2009. PMID: 19702826
-
The effect of dehydroepiandrosterone supplementation on ovarian response is associated with androgen receptor in diminished ovarian reserve women.J Ovarian Res. 2017 May 4;10(1):32. doi: 10.1186/s13048-017-0326-3. J Ovarian Res. 2017. PMID: 28472976 Free PMC article. Clinical Trial.
-
Dehydroepiandrosterone improves the ovarian reserve of women with diminished ovarian reserve and is a potential regulator of the immune response in the ovaries.Biosci Trends. 2015 Dec;9(6):350-9. doi: 10.5582/bst.2015.01154. Biosci Trends. 2015. PMID: 26781792 Review.
-
Pharmacology and therapeutic effects of dehydroepiandrosterone in older subjects.Drugs Aging. 2003;20(13):949-67. doi: 10.2165/00002512-200320130-00001. Drugs Aging. 2003. PMID: 14561100 Review.
Cited by
-
hUC-MSC Combined with DHEA Alleviates Ovarian Senescence in Naturally Aging Mice through Enhancing Antioxidant Capacity and Inhibiting Inflammatory Response.Stem Cells Int. 2024 Jul 30;2024:3100942. doi: 10.1155/2024/3100942. eCollection 2024. Stem Cells Int. 2024. PMID: 39108701 Free PMC article.
-
DHEA down-regulates mitochondrial dynamics and promotes apoptosis of lung adenocarcinoma cells through FASTKD2.J Cancer. 2024 Feb 24;15(8):2110-2122. doi: 10.7150/jca.93373. eCollection 2024. J Cancer. 2024. PMID: 38495508 Free PMC article.
-
Fetal Zone Steroids Show Discrete Effects on Hyperoxia-Induced Attenuation of Migration in Cultured Oligodendrocyte Progenitor Cells.Oxid Med Cell Longev. 2022 May 9;2022:2606880. doi: 10.1155/2022/2606880. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35585881 Free PMC article.
-
Menopause, androgens, and cardiovascular ageing: a narrative review.Ther Adv Endocrinol Metab. 2022 Oct 28;13:20420188221129946. doi: 10.1177/20420188221129946. eCollection 2022. Ther Adv Endocrinol Metab. 2022. PMID: 36325501 Free PMC article. Review.
References
-
- Trainor BC, Nelson RJ. Neuroendocrinology of aggression [Internet]. In: Handbook of Neuroendocrinology. Academic Press. 2012:509–520.
-
- Young J, Couzinet B, Nahoul K, S Brailly, Chanson P, Baulieu EE, Schaison G. Panhypopituitarism as a model to study the metabolism of dehydroepiandrosterone (DHEA) in humans. J Clin Endocrinol Metab. 1997;82:2578–2585. - PubMed
-
- Zapata E, Ventura JL, De La Cruz K, Rodriguez E, Damián P, Massó F, Montaño LF, López-Marure R. Dehydroepiandrosterone inhibits the proliferation of human umbilical vein endothelial by enhancing the expression of p53 and p21, restricting the phosphorylation of retinoblastoma protein, and is androgen- and estrogen-receptor independent. FEBS J. 2005;272:1343–1353. - PubMed
-
- Ohlsson C, Vandenput L. DHEA and mortality: What is the nature of the association? J Steroid Biochem Mol Biol. 2015;145:248–253. - PubMed
LinkOut - more resources
Full Text Sources
