Gene Therapy in the Anterior Eye Segment
- PMID: 33902406
- PMCID: PMC8531184
- DOI: 10.2174/1566523221666210423084233
Gene Therapy in the Anterior Eye Segment
Abstract
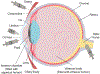
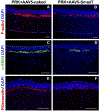
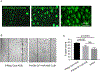
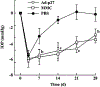
This review provides comprehensive information about the advances in gene therapy in the anterior segment of the eye, including cornea, conjunctiva, lacrimal gland, and trabecular meshwork. We discuss gene delivery systems, including viral and non-viral vectors as well as gene editing techniques, mainly CRISPR-Cas9, and epigenetic treatments, including antisense and siRNA therapeutics. We also provide a detailed analysis of various anterior segment diseases where gene therapy has been tested with corresponding outcomes. Disease conditions include corneal and conjunctival fibrosis and scarring, corneal epithelial wound healing, corneal graft survival, corneal neovascularization, genetic corneal dystrophies, herpetic keratitis, glaucoma, dry eye disease, and other ocular surface diseases. Although most of the analyzed results on the use and validity of gene therapy at the ocular surface have been obtained in vitro or using animal models, we also discuss the available human studies. Gene therapy approaches are currently considered very promising as emerging future treatments of various diseases, and this field is rapidly expanding.
Keywords: CRISPR-Cas9; Gene therapy; adeno-associated virus; adenovirus; antisense; cornea; corneal dystrophy; corneal neovascularization; corneal wound healing; drug delivery; dry eye; glaucoma; graft survival; keratitis; lentivirus; nanoconstruct; non-viral vector; retrovirus; siRNA.
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.net.
Conflict of interest statement
CONFLICT OF INTEREST
AVL is an officer and stockholder of Arrogene Nanotechnology, Inc., 8560 West Sunset Boulevard, Suite 424, Los Angeles, CA 90069, USA.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

