Trastuzumab administration during pregnancy: an update
- PMID: 33902516
- PMCID: PMC8074427
- DOI: 10.1186/s12885-021-08162-3
Trastuzumab administration during pregnancy: an update
Erratum in
-
Correction to: Trastuzumab administration during pregnancy: an update.BMC Cancer. 2021 Dec 17;21(1):1340. doi: 10.1186/s12885-021-09087-7. BMC Cancer. 2021. PMID: 34920709 Free PMC article. No abstract available.
Abstract
Background: Over than one third (28-58%) of pregnancy-associated breast cancer (PABC) cases are characterized by positive epidermal growth factor receptor 2-positive (HER2) expression. Trastuzumab anti-HER2 monoclonal antibody is still the benchmark treatment of HER2-positive breast tumors. However, FDA has categorized Trastuzumab as a category D drug for pregnant patients with breast cancer. This systemic review aims to synthesize all currently available data of trastuzumab administration during pregnancy and provide an updated view of the effect of trastuzumab on fetal and maternal outcome.
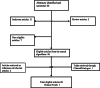
Methods: Eligible articles were identified by a search of MEDLINE bibliographic database and ClinicalTrials.gov for the period up to 01/09/2020; The algorithm consisted of a predefined combination of the words "breast", "cancer", "trastuzumab" and "pregnancy". This study was performed in accordance with the PRISMA guidelines.
Results: A total of 28 eligible studies were identified (30 patients, 32 fetuses). In more than half of cases, trastuzumab was administered in the metastatic setting. The mean duration of trastuzumab administration during gestation was 15.7 weeks (SD: 10.8; median: 17.5; range: 1-32). Oligohydramnios or anhydramnios was the most common (58.1%) adverse event reported in all cases. There was a statistically significant decrease in oligohydramnios/anhydramnios incidence in patients receiving trastuzumab only during the first trimester (P = 0.026, Fisher's exact test). In 43.3% of cases a completely healthy neonate was born. 41.7% of fetuses exposed to trastuzumab during the second and/or third trimester were born completely healthy versus 75.0% of fetuses exposed exclusively in the first trimester. All mothers were alive at a median follow-up of 47.0 months (ranging between 9 and 100 months). Of note, there were three cases (10%) of cardiotoxicity and decreased ejection fraction during pregnancy.
Conclusions: Overall, treatment with trastuzumab should be postponed until after delivery, otherwise pregnancy should be closely monitored.
Keywords: Breast cancer; Gestation; Oligohydramnios; Pregnancy; Trastuzumab; her2.
Conflict of interest statement
MAD has received honoraria from participation in advisory boards from Amgen, Bristol-Myers-Squibb, Celgene, Janssen, Takeda. FZ has received honoraria for lectures and has served in an advisory role for Astra-Zeneca, Eli-Lilly, Merck, Novartis, Pfizer, and Roche. The remaining authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous