Association of plasma mitochondrial DNA with COPD severity and progression in the SPIROMICS cohort
- PMID: 33902556
- PMCID: PMC8074408
- DOI: 10.1186/s12931-021-01707-x
Association of plasma mitochondrial DNA with COPD severity and progression in the SPIROMICS cohort
Abstract
Background: There is a lack of mechanism-driven, clinically relevant biomarkers in chronic obstructive pulmonary disease (COPD). Mitochondrial dysfunction, a proposed disease mechanism in COPD, is associated with the release of mitochondrial DNA (mtDNA), but plasma cell-free mtDNA has not been previously examined prospectively for associations with clinical COPD measures.
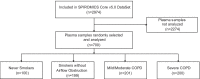
Methods: P-mtDNA, defined as copy number of mitochondrially-encoded NADH dehydrogenase-1 (MT-ND1) gene, was measured by real-time quantitative PCR in 700 plasma samples from participants enrolled in the Subpopulations and Intermediate Outcome Measures in COPD Study (SPIROMICS) cohort. Associations between p-mtDNA and clinical disease parameters were examined, adjusting for age, sex, smoking status, and for informative loss to follow-up.
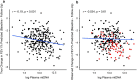
Results: P-mtDNA levels were higher in participants with mild or moderate COPD, compared to smokers without airflow obstruction, and to participants with severe COPD. Baseline increased p-mtDNA levels were associated with better CAT scores in female smokers without airflow obstruction and female participants with mild or moderate COPD on 1-year follow-up, but worse 6MWD in females with severe COPD. Higher p-mtDNA levels were associated with better 6MWD in male participants with severe COPD. These associations were no longer significant after adjusting for informative loss to follow-up.
Conclusion: In this study, p-mtDNA levels associated with baseline COPD status but not future changes in clinical COPD measures after accounting for informative loss to follow-up. To better characterize mitochondrial dysfunction as a potential COPD endotype, these results should be confirmed and validated in future studies.
Trial registration: ClinicalTrials.gov NCT01969344 (SPIROMICS).
Keywords: COPD; Mitochondrial dysfunction; SPIROMICS; mtDNA.
Conflict of interest statement
IB reports grants from NHLBI, during the conduct of the study; grants and personal fees from Theravance and Mylan, grants from Amgen, personal fees from Astra Zeneca, personal fees from GSK, personal fees from Boehringer Ingelheim, personal fees from Verona Pharma, personal fees from Grifols, outside the submitted work. AMKC is a cofounder, stockholder, and serves on the Scientific Advisory Board for Proterris, which develops therapeutic uses for carbon monoxide. A.M.K.C. also has a use patent on CO. A.M.K.C. served as a consultant for Teva Pharmaceuticals in July 2018. MEC reports grants from NIH, during the conduct of the study. DJC reports grants from NHLBI, grants from COPD Foundation, during the conduct of the study. GJC reports grants and personal fees from Galaxo Smith Kline, grants and personal fees from Boehringer Ingelheim, grants and personal fees from Chiesi, grants and personal fees from Mereo, personal fees from Verona, grants and personal fees from Astra Zeneca, grants and personal fees from Pulmonx, grants and personal fees from Pneumrx, personal fees from BTG, grants and personal fees from Olympus, grants and personal fees from Broncus, personal fees from EOLO, personal fees from NGM, grants and personal fees from Lungpacer, grants from Alung, grants and personal fees from Nuvaira, grants and personal fees from ResMed, grants and personal fees from Respironics, grants from Fisher Paykel, grants and personal fees from Patara, grants from Galapgos, outside the submitted work. JLC reports a grant from NIH/NHLBI during the conduct of the study; grants from NIH/NHLBI, NIH/NIAID, the Department of Veterans Affairs, and the Department of Defense, outside the submitted work; and personal fees from AstraZeneca, Ltd. and Novartis AG, outside the submitted work. MKH reports grants from NHLBI, during the conduct of the study; personal fees from GlaxoSmithKline, personal fees from Boehringer Ingelheim, personal fees from AstraZeneca, personal fees from Merck, personal fees from Mylan, other from Sunovion, other from Novartis, outside the submitted work. NHH reports grants and personal fees from AstraZeneca, grants from Boehringer Ingelheim, grants from NIH, grants from COPD Foundation, personal fees from Mylan, outside the submitted work. WWL reports grants from National Institutes of Health, non-financial support from Pulmonx, personal fees from Konica Minolta, outside the submitted work. FJM reports personal fees and non-financial support from American College of Chest Physicians, personal fees and non-financial support from AstraZeneca, personal fees and non-financial support from Boehringer Ingelheim, non-financial support from ProterrixBio, personal fees from Columbia University, personal fees and non-financial support from ConCert, personal fees and non-financial support from Genentech, personal fees and non-financial support from GlaxoSmithKline, personal fees and non-financial support from Inova Fairfax Health System, personal fees from Integritas, personal fees from MD Magazine, personal fees from Methodist Hospital Brooklyn, personal fees and non-financial support from Miller Communicatinos, personal fees and non-financial support from National Association for Continuing Education, personal fees and non-financial support from Novartis, personal fees from New York University, personal fees and non-financial support from Pearl Pharmaceuticals, personal fees and non-financial support from PeerView Communications, personal fees and non-financial support from Prime Communications, personal fees and non-financial support from Puerto Rican Respiratory Society, personal fees and non-financial support from Chiesi, personal fees and non-financial support from Sunovion, personal fees and non-financial support from Theravance, personal fees from UpToDate, personal fees from WebMD/MedScape, personal fees from Western Connecticut Health Network, other from Afferent/Merck, non-financial support from Gilead, non-financial support from Nitto, personal fees from Patara/Respivant, personal fees from PlatformIQ, personal fees and non-financial support from Potomac, other from Biogen, personal fees and non-financial support from University of Alabama Birmingham, other from Veracyte, non-financial support from Zambon, personal fees from American Thoracic Society, grants from NIH, personal fees and non-financial support from Physicians Education Resource, personal fees from Rockpointe, other from Prometic, personal fees from Rare Disease Healthcare Communications, other from Bayer, other from Bridge Biotherapeutics, personal fees and non-financial support from Canadian Respiratory Network, other from ProMedior, personal fees and non-financial support from Teva, personal fees from France Foundation, personal fees and non-financial support from Dartmouth, outside the submitted work. RP reports grants from NHLBI, grants from COPD Foundation, during the conduct of the study; grants from US Department of Veterans Affairs, outside the submitted work. SPP reports personal fees from AstraZeneca, personal fees from Novartis, personal fees from TEVA, personal fees from Wolters Kluwer—UpToDate, personal fees from Palladian Partners, NIAID, personal fees from Syneos Health, personal fees from Syneos Health, personal fees from Genentech, personal fees from GSK, personal fees from Integrity CE, personal fees from Respiratory Medicine—Elsevier, outside the submitted work. NP reports grants from NIH, during the conduct of the study. JMW reports grants from NIH/NHLBI, during the conduct of the study; grants from NIH/NCATS, grants from NIH/NHLBI, grants from Vertex Pharmaceuticals, Inc, grants from Bayer AG, grants from ARCUS-MED, LLC, grants from Mereo BioPharma, other from AstraZeneca, other from GlaxoSmithKline, other from Boehringer Ingelheim, other from Takeda, outside the submitted work. PGW reports grants from NIH, during the conduct of the study; personal fees from Sanofi, personal fees from Regeneron, personal fees from Glenmark, personal fees from Theravance, personal fees from GSK, personal fees from NGM Pharma, personal fees from Genentech, outside the submitted work. All other authors report no competing interests.
Figures

Similar articles
-
Association of urine mitochondrial DNA with clinical measures of COPD in the SPIROMICS cohort.JCI Insight. 2020 Feb 13;5(3):e133984. doi: 10.1172/jci.insight.133984. JCI Insight. 2020. PMID: 31895696 Free PMC article.
-
Anemia and Adverse Outcomes in a Chronic Obstructive Pulmonary Disease Population with a High Burden of Comorbidities. An Analysis from SPIROMICS.Ann Am Thorac Soc. 2018 Jun;15(6):710-717. doi: 10.1513/AnnalsATS.201708-687OC. Ann Am Thorac Soc. 2018. PMID: 30726108 Free PMC article.
-
Airway mucin MUC5AC and MUC5B concentrations and the initiation and progression of chronic obstructive pulmonary disease: an analysis of the SPIROMICS cohort.Lancet Respir Med. 2021 Nov;9(11):1241-1254. doi: 10.1016/S2213-2600(21)00079-5. Epub 2021 May 28. Lancet Respir Med. 2021. PMID: 34058148 Free PMC article.
-
Sleep disruption as a predictor of quality of life among patients in the subpopulations and intermediate outcome measures in COPD study (SPIROMICS).Sleep. 2018 May 1;41(5):zsy044. doi: 10.1093/sleep/zsy044. Sleep. 2018. PMID: 29534240 Free PMC article.
-
Chronic obstructive pulmonary disease with mild airflow limitation: current knowledge and proposal for future research - a consensus document from six scientific societies.Int J Chron Obstruct Pulmon Dis. 2017 Aug 29;12:2593-2610. doi: 10.2147/COPD.S132236. eCollection 2017. Int J Chron Obstruct Pulmon Dis. 2017. PMID: 28919728 Free PMC article. Review.
Cited by
-
Cell-free DNA levels associate with COPD exacerbations and mortality.Respir Res. 2024 Jan 18;25(1):42. doi: 10.1186/s12931-023-02658-1. Respir Res. 2024. PMID: 38238743 Free PMC article.
-
Alterations in the molecular control of mitochondrial turnover in COPD lung and airway epithelial cells.Sci Rep. 2024 Feb 27;14(1):4821. doi: 10.1038/s41598-024-55335-8. Sci Rep. 2024. PMID: 38413800 Free PMC article.
-
Associative analysis of multi-omics data indicates that acetylation modification is widely involved in cigarette smoke-induced chronic obstructive pulmonary disease.Front Med (Lausanne). 2023 Jan 12;9:1030644. doi: 10.3389/fmed.2022.1030644. eCollection 2022. Front Med (Lausanne). 2023. PMID: 36714109 Free PMC article.
-
Comparative Assessment of Acute Pulmonary Effects Induced by Heat-Not-Burn Tobacco Aerosol Inhalation in a Murine Model.Int J Mol Sci. 2025 Jan 28;26(3):1135. doi: 10.3390/ijms26031135. Int J Mol Sci. 2025. PMID: 39940903 Free PMC article.
-
Clinicopathological and circulating cell-free DNA profile in myositis associated with anti-mitochondrial antibody.Ann Clin Transl Neurol. 2023 Nov;10(11):2127-2138. doi: 10.1002/acn3.51901. Epub 2023 Sep 18. Ann Clin Transl Neurol. 2023. PMID: 37723899 Free PMC article.
References
-
- GBD 2015 Chronic Respiratory Disease Collaborators. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med. 2017;5(9):691–706. - PMC - PubMed
-
- Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195(5):557–82. - PubMed
-
- Ford ES, Mannino DM, Wheaton AG, Giles WH, Presley-Cantrell L, Croft JB. Trends in the prevalence of obstructive and restrictive lung function among adults in the United States: findings from the National Health and Nutrition Examination surveys from 1988–1994 to 2007–2010. Chest. 2013;143(5):1395–1406. doi: 10.1378/chest.12-1135. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- HHSN268200900017C/HL/NHLBI NIH HHS/United States
- HHSN268200900018C/HL/NHLBI NIH HHS/United States
- HHSN268200900014C/HL/NHLBI NIH HHS/United States
- HHSN268200900020C/HL/NHLBI NIH HHS/United States
- HHSN268200900016C/HL/NHLBI NIH HHS/United States
- HHSN268200900019C/HL/NHLBI NIH HHS/United States
- R01 HL132198/HL/NHLBI NIH HHS/United States
- HHSN268200900015C/HL/NHLBI NIH HHS/United States
- U01 HL137880/HL/NHLBI NIH HHS/United States
- T32 HL134629/HL/NHLBI NIH HHS/United States
- U24 HL141762/HL/NHLBI NIH HHS/United States
- HHSN268200900013C/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

