Ischaemic postconditioning reduces apoptosis in experimental jejunal ischaemia in horses
- PMID: 33902575
- PMCID: PMC8077964
- DOI: 10.1186/s12917-021-02877-y
Ischaemic postconditioning reduces apoptosis in experimental jejunal ischaemia in horses
Abstract
Background: Ischaemic postconditioning (IPoC) refers to brief periods of reocclusion of blood supply following an ischaemic event. This has been shown to ameliorate ischaemia reperfusion injury in different tissues, and it may represent a feasible therapeutic strategy for ischaemia reperfusion injury following strangulating small intestinal lesions in horses. The objective of this study was to assess the degree cell death, inflammation, oxidative stress, and heat shock response in an equine experimental jejunal ischaemia model with and without IPoC.
Methods: In this randomized, controlled, experimental in vivo study, 14 horses were evenly assigned to a control group and a group subjected to IPoC. Under general anaesthesia, segmental ischaemia with arterial and venous occlusion was induced in 1.5 m jejunum. Following ischaemia, the mesenteric vessels were repeatedly re-occluded in group IPoC only. Full thickness intestinal samples and blood samples were taken at the end of the pre-ischaemia period, after ischaemia, and after 120 min of reperfusion. Immunohistochemical staining or enzymatic assays were performed to determine the selected variables.
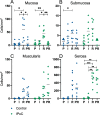
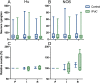
Results: The mucosal cleaved-caspase-3 and TUNEL cell counts were significantly increased after reperfusion in the control group only. The cleaved-caspase-3 cell count was significantly lower in group IPoC after reperfusion compared to the control group. After reperfusion, the tissue myeloperoxidase activity and the calprotectin positive cell counts in the mucosa were increased in both groups, and only group IPoC showed a significant increase in the serosa. Tissue malondialdehyde and superoxide dismutase as well as blood lactate levels showed significant progression during ischaemia or reperfusion. The nuclear immunoreactivity of Heat shock protein-70 increased significantly during reperfusion. None of these variables differed between the groups. The neuronal cell counts in the myenteric plexus ganglia were not affected by the ischaemia model.
Conclusions: A reduced apoptotic cell count was found in the group subjected to IPoC. None of the other tested variables were significantly affected by IPoC. Therefore, the clinical relevance and possible protective mechanism of IPoC in equine intestinal ischaemia remains unclear. Further research on the mechanism of action and its effect in clinical cases of strangulating colic is needed.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






Similar articles
-
The effect of ischaemic postconditioning on mucosal integrity and function in equine jejunal ischaemia.Equine Vet J. 2022 Mar;54(2):427-437. doi: 10.1111/evj.13450. Epub 2021 May 18. Equine Vet J. 2022. PMID: 34003501
-
Ischemic postconditioning attenuate reperfusion injury of small intestine: impact of mitochondrial permeability transition.Transplantation. 2013 Feb 27;95(4):559-65. doi: 10.1097/TP.0b013e31827e6b02. Transplantation. 2013. PMID: 23423267
-
Ischaemic preconditioning and pharmacological preconditioning with dexmedetomidine in an equine model of small intestinal ischaemia-reperfusion.PLoS One. 2020 Apr 29;15(4):e0224720. doi: 10.1371/journal.pone.0224720. eCollection 2020. PLoS One. 2020. PMID: 32348301 Free PMC article.
-
Protection against renal ischemia-reperfusion injury by ischemic postconditioning.Transplantation. 2013 Jun 15;95(11):1299-305. doi: 10.1097/TP.0b013e318281b934. Transplantation. 2013. PMID: 23519023 Review.
-
Intestinal oxygen utilisation and cellular adaptation during intestinal ischaemia-reperfusion injury.Clin Transl Med. 2025 Jan;15(1):e70136. doi: 10.1002/ctm2.70136. Clin Transl Med. 2025. PMID: 39724463 Free PMC article. Review.
Cited by
-
Adaptive mechanisms in no flow vs. low flow ischemia in equine jejunum epithelium: Different paths to the same destination.Front Vet Sci. 2022 Sep 8;9:947482. doi: 10.3389/fvets.2022.947482. eCollection 2022. Front Vet Sci. 2022. PMID: 36157182 Free PMC article.
-
Fecal Secretory Immunoglobulin A and Lactate Level as a Biomarker of Mucosal Immune Dysfunction in Horses With Colic.J Vet Intern Med. 2025 May-Jun;39(3):e70073. doi: 10.1111/jvim.70073. J Vet Intern Med. 2025. PMID: 40145309 Free PMC article.
-
Hypoxia signaling in the equine small intestine: Expression and distribution of hypoxia inducible factors during experimental ischemia.Front Vet Sci. 2023 Feb 24;10:1110019. doi: 10.3389/fvets.2023.1110019. eCollection 2023. Front Vet Sci. 2023. PMID: 36908508 Free PMC article.
-
Low Flow versus No Flow: Ischaemia Reperfusion Injury Following Different Experimental Models in the Equine Small Intestine.Animals (Basel). 2022 Aug 22;12(16):2158. doi: 10.3390/ani12162158. Animals (Basel). 2022. PMID: 36009747 Free PMC article.
References
-
- Auer JA, Stick JA, Kuemmerle JM, Prange T. Equine Surgery. 5. St. Louis: Elsevier Health Sciences; 2019.
-
- Blikslager AT. The equine acute abdomen: John Wiley & Sons. 2017.
-
- Park P, Haglund U, Bulkley G, Fält K. The sequence of development of intestinal tissue injury after strangulation ischemia and reperfusion. Surgery. 1990;107(5):574–580. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

