Efficacy and safety of nintedanib in patients with idiopathic pulmonary fibrosis who are elderly or have comorbidities
- PMID: 33902584
- PMCID: PMC8073950
- DOI: 10.1186/s12931-021-01695-y
Efficacy and safety of nintedanib in patients with idiopathic pulmonary fibrosis who are elderly or have comorbidities
Abstract
Background: Idiopathic pulmonary fibrosis (IPF) predominantly affects individuals aged > 60 years who have several comorbidities. Nintedanib is an approved treatment for IPF, which reduces the rate of decline in forced vital capacity (FVC). We assessed the efficacy and safety of nintedanib in patients with IPF who were elderly and who had multiple comorbidities.
Methods: Data were pooled from five clinical trials in which patients were randomised to receive nintedanib 150 mg twice daily or placebo. We assessed outcomes in subgroups by age < 75 versus ≥ 75 years, by < 5 and ≥ 5 comorbidities, and by Charlson Comorbidity Index (CCI) ≤ 3 and > 3 at baseline.
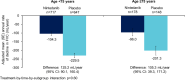
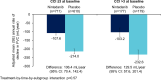
Results: The data set comprised 1690 patients. Nintedanib reduced the rate of decline in FVC (mL/year) over 52 weeks versus placebo in patients aged ≥ 75 years (difference: 105.3 [95% CI 39.3, 171.2]) (n = 326) and < 75 years (difference 125.2 [90.1, 160.4]) (n = 1364) (p = 0.60 for treatment-by-time-by-subgroup interaction), in patients with < 5 comorbidities (difference: 107.9 [95% CI 65.0, 150.9]) (n = 843) and ≥ 5 comorbidities (difference 139.3 [93.8, 184.8]) (n = 847) (p = 0.41 for treatment-by-time-by-subgroup interaction) and in patients with CCI score ≤ 3 (difference: 106.4 [95% CI 70.4, 142.4]) (n = 1330) and CCI score > 3 (difference: 129.5 [57.6, 201.4]) (n = 360) (p = 0.57 for treatment-by-time-by-subgroup interaction). The adverse event profile of nintedanib was generally similar across subgroups. The proportion of patients with adverse events leading to treatment discontinuation was greater in patients aged ≥ 75 years than < 75 years in both the nintedanib (26.4% versus 16.0%) and placebo (12.2% versus 10.8%) groups. Similarly the proportion of patients with adverse events leading to treatment discontinuation was greater in patients with ≥ 5 than < 5 comorbidities (nintedanib: 20.5% versus 15.7%; placebo: 12.1% versus 10.0%).
Conclusions: Our findings suggest that the effect of nintedanib on reducing the rate of FVC decline is consistent across subgroups based on age and comorbidity burden. Proactive management of adverse events is important to reduce the impact of adverse events and help patients remain on therapy.
Trial registration: ClinicalTrials.gov NCT00514683, NCT01335464, NCT01335477, NCT02788474, NCT01979952.
Conflict of interest statement
IG reports personal fees from Boehringer Ingelheim, Roche, Menarini, AdAlta, Pulmotect and Accendatech. FB reports personal fees and non-financial support from Boehringer Ingelheim, Roche, Galapagos, Fujirebio, Bristol-Myers Squibb and GlaxoSmithKline. EBa reports no disclosures. MG has served on advisory boards for Bellerophon Therapeutics, Boehringer Ingelheim and Genentech-Roche. FC reports no disclosures. WS, MQ and LO are employees of Boehringer Ingelheim. EBe reports grants and personal fees from Boehringer Ingelheim and Roche.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical