Elucidation of SIRT-1/PGC-1α-associated mitochondrial dysfunction and autophagy in nonalcoholic fatty liver disease
- PMID: 33902605
- PMCID: PMC8077826
- DOI: 10.1186/s12944-021-01461-5
Elucidation of SIRT-1/PGC-1α-associated mitochondrial dysfunction and autophagy in nonalcoholic fatty liver disease
Abstract
Background: Nonalcoholic fatty liver disease (NAFLD) can lead to chronic liver diseases associated with mitochondrial damages. However, the exact mechanisms involved in the etiology of the disease are not clear.
Methods: To gain new insights, the changes affecting sirtuin 1 (SIRT-1) during liver fat accumulation was investigated in a NAFLD mouse model. In addition, the in vitro research investigated the regulation operated by SIRT-1 on mitochondrial structures, biogenesis, functions, and autophagy.
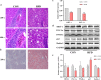
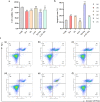
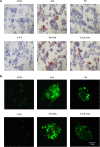
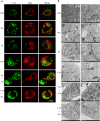
Results: In mice NAFLD, high-fat-diet (HFD) increased body weight gain, upregulated serum total cholesterol, triglycerides, aspartate aminotransferase, alanine aminotransferase, blood glucose, insulin levels, and liver malondialdehyde, and decreased liver superoxide dismutase activity. In liver, the levels of SIRT-1 and peroxisome proliferator-activated receptor-gamma coactivator -1α (PGC-1α) decreased. The expression of peroxisome proliferator-activated receptor-α and Beclin-1 proteins was also reduced, while p62/SQSTM1 expression increased. These results demonstrated SIRT-1 impairment in mouse NAFLD. In a well-established NAFLD cell model, exposure of the HepG2 hepatocyte cell line to oleic acid (OA) for 48 h caused viability reduction, apoptosis, lipid accumulation, and reactive oxygen species production. Disturbance of SIRT-1 expression affected mitochondria. Pre-treatment with Tenovin-6, a SIRT-1 inhibitor, aggravated the effect of OA on hepG2, while this effect was reversed by CAY10602, a SIRT-1 activator. Further investigation demonstrated that SIRT-1 activity was involved in mitochondrial biogenesis through PGC-1α and participated to the balance of autophagy regulatory proteins.
Conclusion: In conclusion, in high-fat conditions, SIRT-1 regulates multiple cellular properties by influencing on mitochondrial physiology and lipid autophagy via the PGC-1α pathway. The SIRT-1/PGC-1α pathway could be targeted to develop new NAFLD therapeutic strategies.
Keywords: Mitochondrial autophagy; Mitochondrial dysfunction; Lipid autophagy; Mitochondrial physiology; Nonalcoholic fatty liver disease; Peroxisome proliferator-activated receptor-gamma coactivator -1a; Sirtuin 1.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Progress in Nonalcoholic Fatty Liver Disease: SIRT Family Regulates Mitochondrial Biogenesis.Biomolecules. 2022 Aug 5;12(8):1079. doi: 10.3390/biom12081079. Biomolecules. 2022. PMID: 36008973 Free PMC article. Review.
-
Acute Elevated Resistin Exacerbates Mitochondrial Damage and Aggravates Liver Steatosis Through AMPK/PGC-1α Signaling Pathway in Male NAFLD Mice.Horm Metab Res. 2021 Feb;53(2):132-144. doi: 10.1055/a-1293-8250. Epub 2020 Dec 10. Horm Metab Res. 2021. PMID: 33302316
-
LB100 ameliorates nonalcoholic fatty liver disease via the AMPK/Sirt1 pathway.World J Gastroenterol. 2019 Dec 7;25(45):6607-6618. doi: 10.3748/wjg.v25.i45.6607. World J Gastroenterol. 2019. PMID: 31832001 Free PMC article.
-
Mitochondrial biogenesis is impaired in osteoarthritis chondrocytes but reversible via peroxisome proliferator-activated receptor γ coactivator 1α.Arthritis Rheumatol. 2015 May;67(8):2141-53. doi: 10.1002/art.39182. Arthritis Rheumatol. 2015. PMID: 25940958 Free PMC article.
-
Role of mitochondria in nonalcoholic fatty liver disease.Int J Mol Sci. 2014 May 15;15(5):8713-42. doi: 10.3390/ijms15058713. Int J Mol Sci. 2014. PMID: 24837835 Free PMC article. Review.
Cited by
-
Novel Role of the SIRT1 in Endocrine and Metabolic Diseases.Int J Biol Sci. 2023 Jan 1;19(2):484-501. doi: 10.7150/ijbs.78654. eCollection 2023. Int J Biol Sci. 2023. PMID: 36632457 Free PMC article. Review.
-
The sirtuin family in health and disease.Signal Transduct Target Ther. 2022 Dec 29;7(1):402. doi: 10.1038/s41392-022-01257-8. Signal Transduct Target Ther. 2022. PMID: 36581622 Free PMC article. Review.
-
Progress in Nonalcoholic Fatty Liver Disease: SIRT Family Regulates Mitochondrial Biogenesis.Biomolecules. 2022 Aug 5;12(8):1079. doi: 10.3390/biom12081079. Biomolecules. 2022. PMID: 36008973 Free PMC article. Review.
-
CTRP3 alleviates mitochondrial dysfunction and oxidative stress injury in pathological cardiac hypertrophy by activating UPRmt via the SIRT1/ATF5 axis.Cell Death Discov. 2024 Jan 26;10(1):53. doi: 10.1038/s41420-024-01813-x. Cell Death Discov. 2024. PMID: 38278820 Free PMC article.
-
Computational Analysis and Experimental Data Exploring the Role of Hesperetin in Ameliorating ADHD and SIRT1/Nrf2/Keap1/OH-1 Signaling.Int J Mol Sci. 2024 Aug 27;25(17):9284. doi: 10.3390/ijms25179284. Int J Mol Sci. 2024. PMID: 39273230 Free PMC article.
References
-
- Wohua Z, Weiming X. Weiming, Glutaredoxin 2 (GRX2) deficiency exacerbates high fat diet (HFD)-induced insulin resistance, inflammation and mitochondrial dysfunction in brain injury: a mechanism involving GSK-3beta. Biomed Pharmacother. 2019;118:e108940. doi: 10.1016/j.biopha.2019.108940. - DOI - PubMed
-
- Ferey JLA, Boudoures AL, Reid M, Drury A, Scheaffer S, Modi Z, Kovacs A, Pietka T, DeBosch BJ, Thompson MD, Diwan A, Moley KH. A maternal high-fat, high-sucrose diet induces transgenerational cardiac mitochondrial dysfunction independently of maternal mitochondrial inheritance. Am J Physiol Heart Circ Physiol. 2019;316:1202–1210. doi: 10.1152/ajpheart.00013.2019. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

