Cold-inducible RNA-binding protein (CIRP) potentiates uric acid-induced IL-1β production
- PMID: 33902703
- PMCID: PMC8074240
- DOI: 10.1186/s13075-021-02508-9
Cold-inducible RNA-binding protein (CIRP) potentiates uric acid-induced IL-1β production
Abstract
Background: Gout is an autoinflammatory disease driven by interleukin-1 (IL-1) induction in response to uric acid crystals. IL-1β production is dependent on inflammasome activation, which requires a priming signal, followed by an activating signal. The cold-inducible RNA-binding protein (CIRP) has been recently identified as a damage-associated molecular pattern (DAMP). In this study, we evaluated the roles of CIRP in monosodium urate (MSU)-mediated IL-1β secretion using human neutrophils.
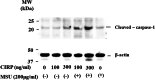
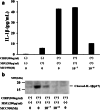
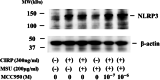
Methods: Human neutrophils were stimulated by MSU in the presence or absence of CIRP priming to determine NLRP3 inflammasome activation and subsequent caspase-1 activation and IL-1β production. Cellular supernatants were analyzed by enzyme-linked immunosorbent assay (ELISA) to determine the presence of IL-1β or caspase-1 (p20). The cellular supernatants and lysates were also analyzed by immunoblotting using anti-cleaved IL-1β or anti-cleaved caspase-1 antibodies.
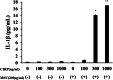
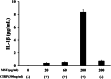
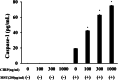
Results: Neither CIRP nor MSU stimulation alone induced sufficient IL-1β secretion from neutrophils. However, MSU stimulation induced IL-1β secretion from CIRP-primed neutrophils in a dose-dependent manner. This MSU-induced IL-1β secretion from CIRP-primed neutrophils was accompanied by the induction of cleaved IL-1β (p17), which was inhibited by the pretreatment of MCC950, a specific inhibitor for NLRP3. Furthermore, cleaved caspase-1 was induced in the cellular lysates of CIRP/MSU-treated neutrophils. Additionally, CIRP stimulation induced the protein expression of pro-IL-1β in neutrophils.
Conclusions: Our data indicate that CIRP, an endogenous stress molecule, triggers uric acid-induced mature IL-1β induction as a priming stimulus for NLRP3 inflammasome in human neutrophils. We propose that CIRP acts as an important proinflammatory stimulant that primes and activates inflammasome and pro-IL-1β processing in response to uric acid in innate immune cells.
Conflict of interest statement
KM has received research grants from Chugai, Pfizer, and AbbVie. The rest of the authors declares that they have no competing interests.
Figures


Similar articles
-
Uric acid-mediated inflammasome activation in IL-6 primed innate immune cells is regulated by baricitinib.Mod Rheumatol. 2021 Jan;31(1):270-275. doi: 10.1080/14397595.2020.1740410. Epub 2020 Mar 30. Mod Rheumatol. 2021. PMID: 32148148
-
NLRP3 inflammasome-mediated neutrophil recruitment and hypernociception depend on leukotriene B(4) in a murine model of gout.Arthritis Rheum. 2012 Feb;64(2):474-84. doi: 10.1002/art.33355. Arthritis Rheum. 2012. PMID: 21952942
-
TNF-α potentiates uric acid-induced interleukin-1β (IL-1β) secretion in human neutrophils.Mod Rheumatol. 2018 May;28(3):513-517. doi: 10.1080/14397595.2017.1369924. Epub 2017 Sep 14. Mod Rheumatol. 2018. PMID: 28880687
-
Genetic and Epigenetic Regulation of the Innate Immune Response to Gout.Immunol Invest. 2023 Apr;52(3):364-397. doi: 10.1080/08820139.2023.2168554. Epub 2023 Feb 6. Immunol Invest. 2023. PMID: 36745138 Review.
-
Beneficial Properties of Phytochemicals on NLRP3 Inflammasome-Mediated Gout and Complication.J Agric Food Chem. 2018 Jan 31;66(4):765-772. doi: 10.1021/acs.jafc.7b05113. Epub 2018 Jan 17. J Agric Food Chem. 2018. PMID: 29293001 Review.
Cited by
-
Extracellular Cold-Inducible RNA-Binding Protein and Hemorrhagic Shock: Mechanisms and Therapeutics.Biomedicines. 2024 Dec 25;13(1):12. doi: 10.3390/biomedicines13010012. Biomedicines. 2024. PMID: 39857596 Free PMC article. Review.
-
RBM3 Promotes Anti-inflammatory Responses in Microglia and Serves as a Neuroprotective Target of Ischemic Stroke.Mol Neurobiol. 2024 Oct;61(10):7384-7402. doi: 10.1007/s12035-024-04052-4. Epub 2024 Feb 22. Mol Neurobiol. 2024. PMID: 38386136
-
Inflammatory Response to Regulated Cell Death in Gout and Its Functional Implications.Front Immunol. 2022 Apr 6;13:888306. doi: 10.3389/fimmu.2022.888306. eCollection 2022. Front Immunol. 2022. PMID: 35464445 Free PMC article. Review.
-
The role of inflammasomes in human diseases and their potential as therapeutic targets.Signal Transduct Target Ther. 2024 Jan 5;9(1):10. doi: 10.1038/s41392-023-01687-y. Signal Transduct Target Ther. 2024. PMID: 38177104 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

