Blocking microglial activation of reactive astrocytes is neuroprotective in models of Alzheimer's disease
- PMID: 33902708
- PMCID: PMC8074239
- DOI: 10.1186/s40478-021-01180-z
Blocking microglial activation of reactive astrocytes is neuroprotective in models of Alzheimer's disease
Abstract
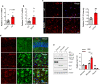
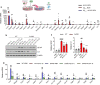
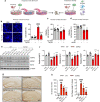
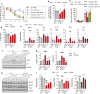
Alzheimer's disease (AD) is the most common cause of age-related dementia. Increasing evidence suggests that neuroinflammation mediated by microglia and astrocytes contributes to disease progression and severity in AD and other neurodegenerative disorders. During AD progression, resident microglia undergo proinflammatory activation, resulting in an increased capacity to convert resting astrocytes to reactive astrocytes. Therefore, microglia are a major therapeutic target for AD and blocking microglia-astrocyte activation could limit neurodegeneration in AD. Here we report that NLY01, an engineered exedin-4, glucagon-like peptide-1 receptor (GLP-1R) agonist, selectively blocks β-amyloid (Aβ)-induced activation of microglia through GLP-1R activation and inhibits the formation of reactive astrocytes as well as preserves neurons in AD models. In two transgenic AD mouse models (5xFAD and 3xTg-AD), repeated subcutaneous administration of NLY01 blocked microglia-mediated reactive astrocyte conversion and preserved neuronal viability, resulting in improved spatial learning and memory. Our study indicates that the GLP-1 pathway plays a critical role in microglia-reactive astrocyte associated neuroinflammation in AD and the effects of NLY01 are primarily mediated through a direct action on Aβ-induced GLP-1R+ microglia, contributing to the inhibition of astrocyte reactivity. These results show that targeting upregulated GLP-1R in microglia is a viable therapy for AD and other neurodegenerative disorders.
Keywords: Alzheimer’s disease; GLP-1 receptor; GLP-1R agonist; Microglia activation; NLY01; Reactive astrocytes.
Conflict of interest statement
J.S.P., K.C.L., E.J.P., and S.L. are employees of D&D Pharmatech. Sa.L., Y.O., S.H.K., and S.L. are employees of Neuraly. M.G.P., H.S.K., V.L.D, T.M.D., and S.L. hold ownership equity of D&D Pharmatech and this arrangement has been reviewed and approved by the Johns Hopkins University in accordance with its conflict of interest policies.
Figures


References
-
- Andrabi SA, Kang HC, Haince JF, Lee YI, Zhang J, Chi Z, West AB, Koehler RC, Poirier GG, Dawson TM, Dawson VL. Iduna protects the brain from glutamate excitotoxicity and stroke by interfering with poly(ADP-ribose) polymer-induced cell death. Nat Med. 2011;17:692–699. doi: 10.1038/nm.2387. - DOI - PMC - PubMed
-
- Bomba M, Ciavardelli D, Silvestri E, Canzoniero LM, Lattanzio R, Chiappini P, Piantelli M, Di Ilio C, Consoli A, Sensi SL. Exenatide promotes cognitive enhancement and positive brain metabolic changes in PS1-KI mice but has no effects in 3xTg-AD animals. Cell Death Dis. 2013;4:e612. doi: 10.1038/cddis.2013.139. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

