Deuterium metabolic imaging - Back to the future
- PMID: 33902815
- PMCID: PMC8083995
- DOI: 10.1016/j.jmr.2021.106932
Deuterium metabolic imaging - Back to the future
Abstract
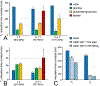
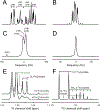
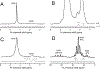
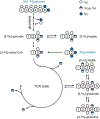
Deuterium metabolic spectroscopy (DMS) and imaging (DMI) have recently been described as simple and robust MR-based methods to map metabolism with high temporal and/or spatial resolution. The metabolic fate of a wide range of suitable deuterated substrates, including glucose and acetate, can be monitored with deuterium MR methods in which the favorable MR characteristics of deuterium prevent many of the complications that hamper other techniques. The short T1 relaxation times lead to good MR sensitivity, while the low natural abundance prevents the need for water or lipid suppression. The sparsity of the deuterium spectra in combination with the low resonance frequency provides relative immunity to magnetic field inhomogeneity. Taken together, these features combine into a highly robust metabolic imaging method that has strong potential to become a dominant MR research tool and a viable clinical imaging modality. This perspective reviews the history of deuterium as a metabolic tracer, the use of NMR as a detection method for deuterium in vitro and in vivo and the recent development of DMS and DMI. Following a review of the NMR characteristics and the biological effects of deuterium, the promising future of DMI is outlined.
Keywords: Deuterium; Glucose; Label loss; Metabolic imaging; Relaxation.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Rothman DL, de Graaf RA, Hyder F, Mason GF, Behar KL, De Feyter HM. In vivo 13C and 1H-[13C] MRS studies of neuroenergetics and neurotransmitter cycling, applications to neurological and psychiatric disease and brain cancer. NMR Biomed 2019;32(10):e4172. - PubMed
-
- Nelson SJ, Kurhanewicz J, Vigneron DB, Larson PE, Harzstark AL, Ferrone M, van Criekinge M, Chang JW, Bok R, Park I, Reed G, Carvajal L, Small EJ, Munster P, Weinberg VK, Ardenkjaer-Larsen JH, Chen AP, Hurd RE, Odegardstuen LI, Robb FJ, Tropp J, Murray JA. Metabolic imaging of patients with prostate cancer using hyperpolarized [1-13C]pyruvate. Science translational medicine 2013;5:198ra108. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

