Uncovering How Auxin Optimizes Root Systems Architecture in Response to Environmental Stresses
- PMID: 33903159
- PMCID: PMC8559545
- DOI: 10.1101/cshperspect.a040014
Uncovering How Auxin Optimizes Root Systems Architecture in Response to Environmental Stresses
Abstract
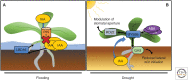
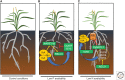
Since colonizing land, plants have developed mechanisms to tolerate a broad range of abiotic stresses that include flooding, drought, high salinity, and nutrient limitation. Roots play a key role acclimating plants to these as their developmental plasticity enables them to grow toward more favorable conditions and away from limiting or harmful stresses. The phytohormone auxin plays a key role translating these environmental signals into developmental outputs. This is achieved by modulating auxin levels and/or signaling, often through cross talk with other hormone signals like abscisic acid (ABA) or ethylene. In our review, we discuss how auxin controls root responses to water, osmotic and nutrient-related stresses, and describe how the synthesis, degradation, transport, and response of this key signaling hormone helps optimize root architecture to maximize resource acquisition while limiting the impact of abiotic stresses.
Copyright © 2021 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures

References
-
- Abas L, Benjamins R, Malenica N, Paciorek T, Wišniewska J, Moulinier-Anzola JC, Sieberer T, Friml J, Luschnig C. 2006. Intracellular trafficking and proteolysis of the Arabidopsis auxin-efflux facilitator PIN2 are involved in root gravitropism. Nat Cell Biol 8: 249–256. 10.1038/ncb1369 - DOI - PubMed
-
- Armstrong W. 1980. Aeration in higher plants. Adv Bot Res 7: 225–332. 10.1016/S0065-2296(08)60089-0 - DOI
-
- Band LR, Wells DM, Larrieu A, Sun J, Middleton AM, French AP, Brunoud G, Sato EM, Wilson MH, Peŕet B, et al. 2012. Root gravitropism is regulated by a transient lateral auxin gradient controlled by a tipping-point mechanism. Proc Natl Acad Sci 109: 4668−4673. 10.1073/pnas.1201498109 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
