Serum Neurofilament Levels and PML Risk in Patients With Multiple Sclerosis Treated With Natalizumab
- PMID: 33903203
- PMCID: PMC8105883
- DOI: 10.1212/NXI.0000000000001003
Serum Neurofilament Levels and PML Risk in Patients With Multiple Sclerosis Treated With Natalizumab
Abstract
Objectives: The study aimed to assess the potential for serum neurofilament light chain (NFL) levels to predict the risk of progressive multifocal leukoencephalopathy (PML) in natalizumab (NTZ)-treated patients with multiple sclerosis (MS) and to discriminate PML from MS relapses.
Methods: NFL levels were measured with single molecule array (Simoa) in 4 cohorts: (1) a prospective cohort of patients with MS who developed PML under NTZ therapy (pre-PML) and non-PML NTZ-treated patients (NTZ-ctr); (2) a cohort of patients whose blood was collected during PML; (3) an independent cohort of non-PML NTZ-treated patients with serum NFL determinations at 2 years (replication cohort); and (4) a cohort of patients whose blood was collected during exacerbations.
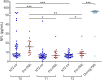
Results: Serum NFL levels were significantly increased after 2 years of NTZ treatment in pre-PML patients compared with NTZ-ctr. The prognostic performance of serum NFL levels to predict PML development at 2 years was similar in the NTZ-ctr group and replication cohort. Serum NFL levels also distinguished PML from MS relapses and were 8-fold higher during PML compared with relapses.
Conclusions: These results support the use of serum NFL levels in clinical practice to identify patients with relapsing-remitting MS at higher PML risk and to differentiate PML from clinical relapses in NTZ-treated patients.
Classification of evidence: This study provides Class I evidence that serum NFL levels can identify NTZ-treated patients with MS who will develop PML with a sensitivity of 67% and specificity of 80%.
Copyright © 2021 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures


References
-
- Polman CH, O'Connor PW, Havrdova E, et al. . A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 2006;354(9):899–910. - PubMed
-
- Bloomgren G, Richman S, Hotermans C, et al. . Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med 2012;366(20):1870–1880. - PubMed
-
- Kappos L, Bates D, Edan G, et al. . Natalizumab treatment for multiple sclerosis: updated recommendations for patient selection and monitoring. Lancet Neurol 2011;10:745–758. - PubMed
-
- Khalil M, Teunissen CE, Otto M, et al. . Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol 2018;14(8):577–589. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
