Multiscale photonic imaging of the native and implanted cochlea
- PMID: 33903231
- PMCID: PMC8106341
- DOI: 10.1073/pnas.2014472118
Multiscale photonic imaging of the native and implanted cochlea
Abstract
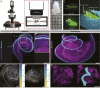
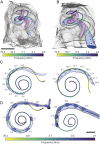
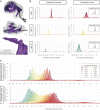
The cochlea of our auditory system is an intricate structure deeply embedded in the temporal bone. Compared with other sensory organs such as the eye, the cochlea has remained poorly accessible for investigation, for example, by imaging. This limitation also concerns the further development of technology for restoring hearing in the case of cochlear dysfunction, which requires quantitative information on spatial dimensions and the sensorineural status of the cochlea. Here, we employed X-ray phase-contrast tomography and light-sheet fluorescence microscopy and their combination for multiscale and multimodal imaging of cochlear morphology in species that serve as established animal models for auditory research. We provide a systematic reference for morphological parameters relevant for cochlear implant development for rodent and nonhuman primate models. We simulate the spread of light from the emitters of the optical implants within the reconstructed nonhuman primate cochlea, which indicates a spatially narrow optogenetic excitation of spiral ganglion neurons.
Keywords: X-ray phase-contrast tomography; cochlear implant; hearing restoration; light-sheet fluorescence microscopy; optogenetics.
Conflict of interest statement
Competing interest statement: T.M. and D.K. are co-founders of OptoGenTech company.
Figures



References
-
- Kral A., Hartmann R., Mortazavi D., Klinke R., Spatial resolution of cochlear implants: The electrical field and excitation of auditory afferents. Hear. Res. 121, 11–28 (1998). - PubMed
-
- Wrobel C., et al. ., Optogenetic stimulation of cochlear neurons activates the auditory pathway and restores auditory-driven behavior in deaf adult gerbils. Sci. Transl. Med. 10, eaao0540 (2018). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

