Higher gametocyte production and mosquito infectivity in chronic compared to incident Plasmodium falciparum infections
- PMID: 33903595
- PMCID: PMC8076179
- DOI: 10.1038/s41467-021-22573-7
Higher gametocyte production and mosquito infectivity in chronic compared to incident Plasmodium falciparum infections
Abstract
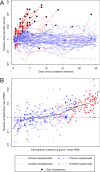
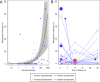
Plasmodium falciparum gametocyte kinetics and infectivity may differ between chronic and incident infections. In the current study, we assess parasite kinetics and infectivity to mosquitoes among children (aged 5-10 years) from Burkina Faso with (a) incident infections following parasite clearance (n = 48) and (b) chronic asymptomatic infections (n = 60). In the incident infection cohort, 92% (44/48) of children develop symptoms within 35 days, compared to 23% (14/60) in the chronic cohort. All individuals with chronic infection carried gametocytes or developed them during follow-up, whereas only 35% (17/48) in the incident cohort produce gametocytes before becoming symptomatic and receiving treatment. Parasite multiplication rate (PMR) and the relative abundance of ap2-g and gexp-5 transcripts are positively associated with gametocyte production. Antibody responses are higher and PMR lower in chronic infections. The presence of symptoms and sexual stage immune responses are associated with reductions in gametocyte infectivity to mosquitoes. We observe that most incident infections require treatment before the density of mature gametocytes is sufficient to infect mosquitoes. In contrast, chronic, asymptomatic infections represent a significant source of mosquito infections. Our observations support the notion that malaria transmission reduction may be expedited by enhanced case management, involving both symptom-screening and infection detection.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- World Health Organisation. Measures of Efficacy of Anti-malaria Interventions Against Malaria Transmission (WHO, Geneva, 2010).
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

