Adoption of PCSK9 Inhibitors Among Patients With Atherosclerotic Disease
- PMID: 33904340
- PMCID: PMC8200752
- DOI: 10.1161/JAHA.120.019331
Adoption of PCSK9 Inhibitors Among Patients With Atherosclerotic Disease
Abstract
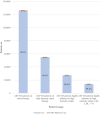
Background PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitors represent a promising class of lipid-lowering therapy, although their use has been limited by cost concerns. Methods and Results A retrospective cohort study was conducted using a nationwide commercial claims database comprising patients with atherosclerotic cardiovascular disease (ASCVD), aged 18 to 64 years. We identified the number of patients with ASCVD started on a PCSK9 inhibitor from the dates of US Food and Drug Administration approval in quarter 3 2015 through quarter 2 2019. Secondary objectives identified the proportions of patients started on a PCSK9 inhibitor in various ASCVD risk groups based on statin use and baseline low-density lipoprotein cholesterol. We identified 126 419 patients with ASCVD on either PCSK9 inhibitor or statin therapy. Among these patients, 1168 (0.9%) filled a prescription for a PCSK9 inhibitor. The number of patients initiating a PCSK9 inhibitor increased from 2 patients in quarter 3 2015 to 119 patients in quarter 2 2019, corresponding to an increase from 0.05% to 2.5% of patients with ASCVD already on statins who started PCSK9 inhibitor therapy. Of patients with ASCVD with high adherence to a high-intensity statin, 13 643 had low-density lipoprotein cholesterol ≥70 mg/dL, and in this subgroup, 119 (0.9%) patients initiated a PCSK9 inhibitor. Conclusions Few patients started PCSK9 inhibitors from 2015 through mid-2019, despite increasing trial evidence of efficacy, guidelines recommending PCSK9 inhibitors in high-risk patients with ASCVD, and price reductions during this period. The magnitude of price reductions may not yet be sufficient to influence use management strategies aimed to limit PCSK9 inhibitor use.
Keywords: PCSK9 inhibitors; access to care; drug adoption; secondary prevention.
Conflict of interest statement
None.
Figures

References
-
- Kazi DS, Moran AE, Coxson PG, Penko J, Ollendorf DA, Pearson SD, Tice JA, Guzman D, Bibbins‐Domingo K. Cost‐effectiveness of PCSK9 inhibitor therapy in patients with heterozygous familial hypercholesterolemia or atherosclerotic cardiovascular disease. JAMA. 2016;316:743–753. DOI: 10.1001/jama.2016.11004. - DOI - PubMed
-
- Arrieta A, Hong JC, Khera R, Virani SS, Krumholz HM, Nassir K. Updated cost‐effectiveness assessments of PCSK9 inhibitors from the perspectives of the health system and private payers: insights derived from the FOURIER trial. JAMA Cardiol. 2017;2:1369–1374. DOI: 10.1001/jamacardio.2017.3655. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

