Memory enhancement by multidomain group cognitive training in patients with Parkinson's disease and mild cognitive impairment: long-term effects of a multicenter randomized controlled trial
- PMID: 33904966
- PMCID: PMC8563628
- DOI: 10.1007/s00415-021-10568-9
Memory enhancement by multidomain group cognitive training in patients with Parkinson's disease and mild cognitive impairment: long-term effects of a multicenter randomized controlled trial
Abstract
Background: Meta-analyses indicate positive effects of cognitive training (CT) in patients with Parkinson's disease (PD), however, most previous studies had small sample sizes and did not evaluate long-term follow-up. Therefore, a multicenter randomized controlled, single-blinded trial (Train-ParC study) was conducted to examine CT effects in PD patients with mild cognitive impairment (PD-MCI). Immediately after CT, an enhancement of executive functions was demonstrated. Here, we present the long-term results 6 and 12 months after CT.
Methods: At baseline, 64 PD-MCI patients were randomized to a multidomain CT group (n = 33) or to a low-intensity physical activity training control group (PT) (n = 31). Both interventions included 90 min training sessions twice a week for 6 weeks. 54 patients completed the 6 months (CT: n = 28, PT: n = 26) and 49 patients the 12 months follow-up assessment (CT: n = 25, PT: n = 24). Primary study outcomes were memory and executive functioning composite scores. Mixed repeated measures ANOVAs, post-hoc t tests and multiple regression analyses were conducted.
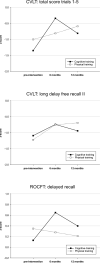
Results: We found a significant time x group interaction effect for the memory composite score (p = 0.006, η2 = 0.214), but not for the executive composite score (p = 0.967, η2 = 0.002). Post-hoc t tests revealed significant verbal and nonverbal memory improvements from pre-intervention to 6 months, but not to 12 months follow-up assessment in the CT group. No significant predictors were found for predicting memory improvement after CT.
Conclusions: This study provides Class 1 evidence that multidomain CT enhances memory functioning in PD-MCI after 6 months but not after 12 months, whereas executive functioning did not change in the long-term.
Clinical trial registration: German Clinical Trials Register (ID: DRKS00010186), 21.3.2016 (The study registration is outlined as retrospective due to an administrative delay. The first patient was enrolled three months after the registration process was started. A formal confirmation of this process from the German Clinical Trials Register can be obtained from the authors.).
Keywords: Cognition; Cognitive training; Long-term effects; Mild cognitive impairment; Non-pharmacological intervention; Parkinson’s disease.
© 2021. The Author(s).
Conflict of interest statement
NS has received grants from the German Federal Ministry of Education and Research. DB has received honoraria from AbbVie, Biogen, BIAL, UCB Pharma GmbH Zambon, and Desitin and grants from Deutsche Forschungsgemeinschaft (DFG), German Parkinson’s Disease Association (dPV), BMBF, Parkinson Fonds Deutschland gGmbH, UCB Pharma GmbH, EU, Novartis Pharma GmbH, Lundbeck, and Damp foundation. She serves as a consultant for Biogen, BIAL, UCB Pharma GmbH, and Zambon. CSchl received funding from the Coppenrath-Foundation, the Parkinson Fonds Germany and honoraria from AbbVie, Wiesbaden, Germany. AKF has received grants from the German Parkinson Society and the German Alzheimer’s Society, as well as honoraria from ProLog Wissen GmbH, Cologne, Germany, pro audito Switzerland, Zürich, Switzerland, Seminar- und Fortbildungszentrum Rheine, Germany, and LOGOMANIA, Fendt & Sax GbR, Munich, Germany. AFK is author of the cognitive training program NEUROvitalis but receives no corresponding honoraria. LW has received honoraria from Meda, Boehringer, Cephalon Pharma, TEVA, Desitin, AbbVie St. Jude Medical/Abbott, and Medtronic and grants from HHU Düsseldorf, DFG Forschergruppe (FOR 1328), ERANET-Neuron/BMBF (TYMON 01EW141), German Parkinson's Disease Association (dPV), Parkinson Fonds Germany, and Hilde Ulrichs Stiftung für Parkinsonforschung. ILS has received grants from International Parkinson Fonds (Deutschland) GmbH (IPD), Johnson & Johnson, European Commission, H2020-TWINN-2015, grants from Michael J. Fox Foundation outside the submitted work. CE has received grants from the German Research Foundation (KFO219, TP 10) and the Medical Faculty of the Philipps University Marburg, Germany, and the German Ministry of Education and Research and honoraria from AbbVie, Wiesbaden, Germany; UCB, Monheim, Germany; Daiichi Sankyo, Munich, Germany; Medtronic, Meerbusch, Germany; Bayer Vital, Leverkusen, Germany; and Bial, Morfelden-Walldorf, Germany. EK has received grants from the German Ministry of Education and Research, ParkinsonFonds Deutschland gGmbH, the German Parkinson Society, the German Alzheimer’s Society; honoraria from: Oticon GmbH, Hamburg, Germany; Lilly Pharma GmbH, Bad Homburg, Germany; Bernafon AG, Bern, Switzerland; Desitin GmbH, Hamburg, Germany. EK is author of the cognitive training program NEUROvitalis but receives no corresponding honoraria. KW serves as a consultant for BIAL. IT, AO, KD, SE, PS, and CSchu declare that there are no conflicts of interest.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical