A Structure-Activity Relationship Study of Bimodal BODIPY-Labeled PSMA-Targeting Bioconjugates
- PMID: 33905162
- PMCID: PMC8453963
- DOI: 10.1002/cmdc.202100210
A Structure-Activity Relationship Study of Bimodal BODIPY-Labeled PSMA-Targeting Bioconjugates
Abstract
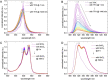
The aim of this study was to identify a high-affinity BODIPY peptidomimetic that targets the prostate-specific membrane antigen (PSMA) as a potential bimodal imaging probe for prostate cancer. For the structure-activity study, several BODIPY (difluoroboron dipyrromethene) derivatives with varying spacers between the BODIPY dye and the PSMA Glu-CO-Lys binding motif were prepared. Corresponding affinities were determined by competitive binding assays in PSMA-positive LNCaP cells. One compound was identified with comparable affinity (IC50 =21.5±0.1 nM) to Glu-CO-Lys-Ahx-HBED-CC (PSMA-11) (IC50 =18.4±0.2 nM). Radiolabeling was achieved by Lewis-acid-mediated 19 F/18 F exchange in moderate molar activities (∼0.7 MBq nmol-1 ) and high radiochemical purities (>99 %) with mean radiochemical yields of 20-30 %. Cell internalization of the 18 F-labeled high-affinity conjugate was demonstrated in LNCaP cells showing gradual increasing PSMA-mediated internalization over time. By fluorescence microscopy, localization of the high-affinity BODIPY-PSMA conjugate was found in the cell membrane at early time points and also in subcellular compartments at later time points. In summary, a high-affinity BODIPY-PSMA conjugate has been identified as a suitable candidate for the development of PSMA-specific dual-imaging agents.
Keywords: BODIPY; PET; PSMA; bimodal imaging; fluorescence imaging.
© 2021 The Authors. ChemMedChem published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

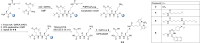

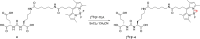



Similar articles
-
Synthesis and pre-clinical evaluation of a new class of high-affinity 18F-labeled PSMA ligands for detection of prostate cancer by PET imaging.Eur J Nucl Med Mol Imaging. 2017 Apr;44(4):647-661. doi: 10.1007/s00259-016-3556-5. Epub 2016 Nov 15. Eur J Nucl Med Mol Imaging. 2017. PMID: 27847991 Free PMC article.
-
A high-affinity [(18)F]-labeled phosphoramidate peptidomimetic PSMA-targeted inhibitor for PET imaging of prostate cancer.Nucl Med Biol. 2015 Oct;42(10):780-7. doi: 10.1016/j.nucmedbio.2015.06.003. Epub 2015 Jun 9. Nucl Med Biol. 2015. PMID: 26169882 Free PMC article.
-
Synthesis and evaluation of a novel urea-based 68Ga-complex for imaging PSMA binding in tumor.Nucl Med Biol. 2018 Apr;59:36-47. doi: 10.1016/j.nucmedbio.2017.12.007. Epub 2017 Dec 27. Nucl Med Biol. 2018. PMID: 29459281
-
Current use of PSMA-PET in prostate cancer management.Nat Rev Urol. 2016 Apr;13(4):226-35. doi: 10.1038/nrurol.2016.26. Epub 2016 Feb 23. Nat Rev Urol. 2016. PMID: 26902337 Review.
-
68Ga-Labeled Prostate-specific Membrane Antigen Ligand Positron Emission Tomography/Computed Tomography for Prostate Cancer: A Systematic Review and Meta-analysis.Eur Urol Focus. 2018 Sep;4(5):686-693. doi: 10.1016/j.euf.2016.11.002. Epub 2016 Nov 15. Eur Urol Focus. 2018. PMID: 28753806
Cited by
-
Asymmetric rotaxanes as dual-modality supramolecular imaging agents for targeting cancer biomarkers.Commun Chem. 2023 Jun 1;6(1):107. doi: 10.1038/s42004-023-00906-5. Commun Chem. 2023. PMID: 37264077 Free PMC article.
-
Development of a homotrimeric PSMA radioligand based on the NOTI chelating platform.EJNMMI Radiopharm Chem. 2024 Dec 11;9(1):84. doi: 10.1186/s41181-024-00314-7. EJNMMI Radiopharm Chem. 2024. PMID: 39661209 Free PMC article.
References
-
- Tipirneni K. E., Rosenthal E. L., Moore L. S., Haskins A. D., Udayakumar N., Jani A. H., Carroll W. R., Morlandt A. B., Bogyo M., Rao J., Warram J. M., Mol. Imaging Biol. 2017, 19, 645–655. - PubMed
-
- Kowada T., Maeda H., Kikuchi K., Chem. Soc. Rev. 2015, 44, 4953–4972. - PubMed
-
- Ntziachristos V., Bremer C., Weissleder R., Eur. Radiol. 2003, 13, 195–208. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

