National Marrow Donor Program-Sponsored Multicenter, Phase II Trial of HLA-Mismatched Unrelated Donor Bone Marrow Transplantation Using Post-Transplant Cyclophosphamide
- PMID: 33905264
- PMCID: PMC8260905
- DOI: 10.1200/JCO.20.03502
National Marrow Donor Program-Sponsored Multicenter, Phase II Trial of HLA-Mismatched Unrelated Donor Bone Marrow Transplantation Using Post-Transplant Cyclophosphamide
Abstract
Purpose: Hematopoietic cell transplantation (HCT) is curative for hematologic disorders, but outcomes are historically inferior when using HLA-mismatched donors. Despite unrelated donor registries listing > 38 million volunteers, 25%-80% of US patients lack an HLA-matched unrelated donor, with significant disparity across ethnic groups. We hypothesized that HCT with a mismatched unrelated donor (MMUD) using post-transplant cyclophosphamide (PTCy), a novel strategy successful in overcoming genetic disparity using mismatched related donors, would be feasible and increase access to HCT.
Patients and methods: We performed a prospective phase II study of MMUD bone marrow HCT with PTCy for patients with hematologic malignancies. The primary end point was 1-year overall survival (OS), hypothesized to be 65% or better. 80 patients enrolled at 11 US transplant centers (December 2016-March 2019). Following myeloablative or reduced-intensity conditioning-based HCT, patients received PTCy on days +3, +4, with sirolimus and mycophenolate mofetil starting on day +5. We compared outcomes to Center for International Blood and Marrow Transplant Research contemporary controls receiving PTCy.
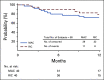
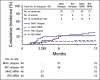
Results: Notably, 48% of patients enrolled were ethnic minorities. 39% of pairs were matched for 4-6 out of 8 HLA alleles. The primary end point was met, with 1-year OS of 76% (90% CI, 67.3 to 83.3) in the entire cohort, and 72% and 79% in the myeloablative and reduced-intensity conditioning strata, respectively. Secondary end points related to engraftment and graft-versus-host-disease were reached. Multivariate analysis comparing the study group with other mismatched HCT controls found no significant differences in OS.
Conclusion: Our prospective study demonstrates the feasibility and effectiveness of HCT with an MMUD in the setting of PTCy. Remarkably, nearly half of the study participants belonged to an ethnic minority population, suggesting this approach may significantly expand access to HCT.
Trial registration: ClinicalTrials.gov NCT02793544.
Conflict of interest statement
Figures


Comment in
-
Increasing Donor Options in Allogenic Hematopoietic Cell Transplantation.J Clin Oncol. 2021 Jun 20;39(18):1951-1954. doi: 10.1200/JCO.21.00622. Epub 2021 May 4. J Clin Oncol. 2021. PMID: 33945301 No abstract available.
References
-
- Peters C, Schrappe M, von Stackelberg A, et al. Stem-cell transplantation in children with acute lymphoblastic leukemia: A prospective international multicenter trial comparing sibling donors with matched unrelated donors—The ALL-SCT-BFM-2003 trial J Clin Oncol 331265–12742015 - PubMed
-
- Besse K, Maiers M, Confer D, et al. On modeling human leukocyte antigen-identical sibling match probability for allogeneic hematopoietic cell transplantation: Estimating the need for an unrelated donor source Biol Blood Marrow Transplant 22410–4172016 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

