Tumor restriction by type I collagen opposes tumor-promoting effects of cancer-associated fibroblasts
- PMID: 33905375
- PMCID: PMC8159701
- DOI: 10.1172/JCI146987
Tumor restriction by type I collagen opposes tumor-promoting effects of cancer-associated fibroblasts
Abstract
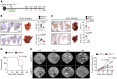
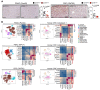
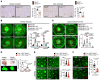
Cancer-associated fibroblasts (CAF) may exert tumor-promoting and tumor-suppressive functions, but the mechanisms underlying these opposing effects remain elusive. Here, we sought to understand these potentially opposing functions by interrogating functional relationships among CAF subtypes, their mediators, desmoplasia, and tumor growth in a wide range of tumor types metastasizing to the liver, the most common organ site for metastasis. Depletion of hepatic stellate cells (HSC), which represented the main source of CAF in mice and patients in our study, or depletion of all CAF decreased tumor growth and mortality in desmoplastic colorectal and pancreatic metastasis but not in nondesmoplastic metastatic tumors. Single-cell RNA-Seq in conjunction with CellPhoneDB ligand-receptor analysis, as well as studies in immune cell-depleted and HSC-selective knockout mice, uncovered direct CAF-tumor interactions as a tumor-promoting mechanism, mediated by myofibroblastic CAF-secreted (myCAF-secreted) hyaluronan and inflammatory CAF-secreted (iCAF-secreted) HGF. These effects were opposed by myCAF-expressed type I collagen, which suppressed tumor growth by mechanically restraining tumor spread, overriding its own stiffness-induced mechanosignals. In summary, mechanical restriction by type I collagen opposes the overall tumor-promoting effects of CAF, thus providing a mechanistic explanation for their dual functions in cancer. Therapeutic targeting of tumor-promoting CAF mediators while preserving type I collagen may convert CAF from tumor promoting to tumor restricting.
Keywords: Cancer; Collagens; Fibrosis; Hepatology; Oncology.
Conflict of interest statement
Figures










References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

