The absence of thrombin-like activity in Bothrops erythromelas venom is due to the deletion of the snake venom thrombin-like enzyme gene
- PMID: 33905416
- PMCID: PMC8078745
- DOI: 10.1371/journal.pone.0248901
The absence of thrombin-like activity in Bothrops erythromelas venom is due to the deletion of the snake venom thrombin-like enzyme gene
Abstract
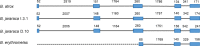
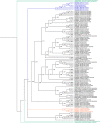
Snake venom thrombin-like enzymes (SVTLEs) are serine proteinases that clot fibrinogen. SVTLEs are distributed mainly in venoms from snakes of the Viperidae family, comprising venomous pit viper snakes. Bothrops snakes are distributed throughout Central and South American and are responsible for most venomous snakebites. Most Bothrops snakes display thrombin-like activity in their venoms, but it has been shown that some species do not present it. In this work, to understand SVTLE polymorphism in Bothrops snake venoms, we studied individual samples from two species of medical importance in Brazil: Bothrops jararaca, distributed in Southeastern Brazil, which displays coagulant activity on plasma and fibrinogen, and Bothrops erythromelas, found in Northeastern Brazil, which lacks direct fibrinogen coagulant activity but shows plasma coagulant activity. We tested the coagulant activity of venoms and the presence of SVTLE genes by a PCR approach. The SVTLE gene structure in B. jararaca is similar to the Bothrops atrox snake, comprising five exons. We could not amplify SVTLE sequences from B. erythromelas DNA, except for a partial pseudogene. These genes underwent a positive selection in some sites, leading to an amino acid sequence diversification, mostly in exon 2. The phylogenetic tree constructed using SVTLE coding sequences confirms that they are related to the chymotrypsin/kallikrein family. Interestingly, we found a B. jararaca specimen whose venom lacked thrombin-like activity, and its gene sequence was a pseudogene with SVTLE structure, presenting nonsense and frameshift mutations. Our results indicate an association of the lack of thrombin-like activity in B. jararaca and B. erythromelas venoms with mutations and deletions of snake venom thrombin-like enzyme genes.
Conflict of interest statement
The authors have declared that no competing interest exist.
Figures

Similar articles
-
Compositional and functional investigation of individual and pooled venoms from long-term captive and recently wild-caught Bothrops jararaca snakes.J Proteomics. 2018 Aug 30;186:56-70. doi: 10.1016/j.jprot.2018.07.007. Epub 2018 Jul 17. J Proteomics. 2018. PMID: 30026101
-
Venomics and antivenomics of Bothrops erythromelas from five geographic populations within the Caatinga ecoregion of northeastern Brazil.J Proteomics. 2015 Jan 30;114:93-114. doi: 10.1016/j.jprot.2014.11.011. Epub 2014 Nov 21. J Proteomics. 2015. PMID: 25462430
-
[Determination of the species origin and thrombin-like enzyme content of Bothrops atrox venom by ultra-high performance liquid chromatography-tandem mass spectrometry based on marker peptide].Se Pu. 2022 Sep;40(9):810-816. doi: 10.3724/SP.J.1123.2021.12020. Se Pu. 2022. PMID: 36156627 Free PMC article. Chinese.
-
The Versatility of Serine Proteases from Brazilian Bothrops Venom: Their Roles in Snakebites and Drug Discovery.Biomolecules. 2025 Jan 21;15(2):154. doi: 10.3390/biom15020154. Biomolecules. 2025. PMID: 40001458 Free PMC article. Review.
-
Practical applications of snake venom toxins in haemostasis.Toxicon. 2005 Jun 15;45(8):1171-81. doi: 10.1016/j.toxicon.2005.02.016. Epub 2005 Apr 7. Toxicon. 2005. PMID: 15922782 Review.
Cited by
-
Clinical and Evolutionary Implications of Dynamic Coagulotoxicity Divergences in Bothrops (Lancehead Pit Viper) Venoms.Toxins (Basel). 2022 Apr 22;14(5):297. doi: 10.3390/toxins14050297. Toxins (Basel). 2022. PMID: 35622544 Free PMC article.
-
Two-Dimensional Blue Native/SDS Polyacrylamide Gel Electrophoresis for Analysis of Brazilian Bothrops Snake Venoms.Toxins (Basel). 2022 Sep 23;14(10):661. doi: 10.3390/toxins14100661. Toxins (Basel). 2022. PMID: 36287928 Free PMC article.
References
-
- Campbell JA, Lamar WW. (2004) The Venomous Reptiles of the Western Hemisphere. 2nd ed. Publishing C, Cornell University Press. 976p.
-
- Gutiérrez JM. Envenenamientos por mordeduras de serpientes en América Latina y el Caribe: una visión integral de carácter regional. Bol Malariol Salud Ambient 2011;51:1–16.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

