Role of Sex-Concordant Gene Expression in the Coevolution of Exaggerated Male and Female Genitalia in a Beetle Group
- PMID: 33905498
- PMCID: PMC8382896
- DOI: 10.1093/molbev/msab122
Role of Sex-Concordant Gene Expression in the Coevolution of Exaggerated Male and Female Genitalia in a Beetle Group
Erratum in
-
Erratum to: Role of Sex-Concordant Gene Expression in the Coevolution of Exaggerated Male and Female Genitalia in a Beetle Group.Mol Biol Evol. 2021 Sep 27;38(10):4658. doi: 10.1093/molbev/msab167. Mol Biol Evol. 2021. PMID: 34338779 Free PMC article. No abstract available.
Abstract
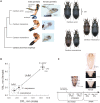
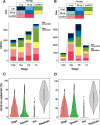
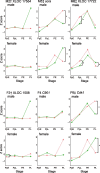
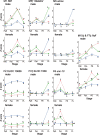
Some sexual traits, including genitalia, have undergone coevolutionary diversification toward exaggerated states in both sexes among closely related species, but the underlying genetic mechanisms that allow correlated character evolution between the sexes are poorly understood. Here, we studied interspecific differences in gene expression timing profiles involved in the correlated evolution of corresponding male and female genital parts in three species of ground beetle in Carabus (Ohomopterus). The male and female genital parts maintain morphological matching, whereas large interspecific variation in genital part size has occurred in the genital coevolution between the sexes toward exaggeration. We analyzed differences in gene expression involved in the interspecific differences in genital morphology using whole transcriptome data from genital tissues during genital morphogenesis. We found that the gene expression variance attributed to sex was negligible for the majority of differentially expressed genes, thus exhibiting sex-concordant expression, although large variances were attributed to stage and species differences. For each sex, we obtained co-expression gene networks and hub genes from differentially expressed genes between species that might be involved in interspecific differences in genital morphology. These gene networks were common to both sexes, and both sex-discordant and sex-concordant gene expression were likely involved in species-specific genital morphology. In particular, the gene expression related to exaggerated genital size showed no significant intersexual differences, implying that the genital sizes in both sexes are controlled by the same gene network with sex-concordant expression patterns, thereby facilitating the coevolution of exaggerated genitalia between the sexes while maintaining intersexual matching.
Keywords: character evolution; interspecific differences; sexual traits; transcriptome; weighted gene co-expression network analysis.
© The Author(s) 2021. Published by Oxford University Press on behalf of the Society for Molecular Biology and Evolution.
Figures
Similar articles
-
Evolutionary changes in gene expression profiles associated with the coevolution of male and female genital parts among closely related ground beetle species.BMC Genomics. 2022 Sep 8;23(1):637. doi: 10.1186/s12864-022-08865-2. BMC Genomics. 2022. PMID: 36076166 Free PMC article.
-
Genetic basis of species-specific genitalia reveals role in species diversification.Sci Adv. 2019 Jun 26;5(6):eaav9939. doi: 10.1126/sciadv.aav9939. eCollection 2019 Jun. Sci Adv. 2019. PMID: 31249868 Free PMC article.
-
Reproductive Character Displacement in Genital Morphology in Ohomopterus Ground Beetles.Am Nat. 2022 Mar;199(3):E76-E90. doi: 10.1086/717864. Epub 2022 Feb 1. Am Nat. 2022. PMID: 35175894
-
Mechanisms and Evidence of Genital Coevolution: The Roles of Natural Selection, Mate Choice, and Sexual Conflict.Cold Spring Harb Perspect Biol. 2015 Jul 1;7(7):a017749. doi: 10.1101/cshperspect.a017749. Cold Spring Harb Perspect Biol. 2015. PMID: 26134314 Free PMC article. Review.
-
The evolution of asymmetric genitalia in spiders and insects.Biol Rev Camb Philos Soc. 2007 Nov;82(4):647-98. doi: 10.1111/j.1469-185X.2007.00029.x. Biol Rev Camb Philos Soc. 2007. PMID: 17944621 Review.
Cited by
-
Evolutionary changes in gene expression profiles associated with the coevolution of male and female genital parts among closely related ground beetle species.BMC Genomics. 2022 Sep 8;23(1):637. doi: 10.1186/s12864-022-08865-2. BMC Genomics. 2022. PMID: 36076166 Free PMC article.
-
Molecular and Developmental Signatures of Genital Size Macro-Evolution in Bugs.Mol Biol Evol. 2022 Oct 7;39(10):msac211. doi: 10.1093/molbev/msac211. Mol Biol Evol. 2022. PMID: 36181434 Free PMC article.
References
-
- Andrews S.2010. FastQC: A quality control tool for high throughput sequence data. Available from: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/
-
- Field CM, Coughlin M, Doberstein S, Marty T, Sullivan W.. 2005. Characterization of anillin mutants reveals essential roles in septin localization and plasma membrane integrity. Development 132:2849–2860. - PubMed
-
- Fisher RA.1930. The genetical theory of natural selection. Oxford: Clarendon Press.
-
- Frisch D, Becker D, Wojewodzic MW.. 2020. Dissecting the transcriptomic basis of phenotypic evolution in an aquatic keystone grazer. Mol Biol Evol. 37:475–487. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources

