SARS-CoV-2 infection of primary human lung epithelium for COVID-19 modeling and drug discovery
- PMID: 33905739
- PMCID: PMC8043574
- DOI: 10.1016/j.celrep.2021.109055
SARS-CoV-2 infection of primary human lung epithelium for COVID-19 modeling and drug discovery
Abstract
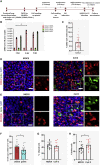
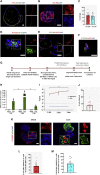
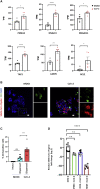
Coronavirus disease 2019 (COVID-19) is the latest respiratory pandemic caused by severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2). Although infection initiates in the proximal airways, severe and sometimes fatal symptoms of the disease are caused by infection of the alveolar type 2 (AT2) cells of the distal lung and associated inflammation. In this study, we develop primary human lung epithelial infection models to understand initial responses of proximal and distal lung epithelium to SARS-CoV-2 infection. Differentiated air-liquid interface (ALI) cultures of proximal airway epithelium and alveosphere cultures of distal lung AT2 cells are readily infected by SARS-CoV-2, leading to an epithelial cell-autonomous proinflammatory response with increased expression of interferon signaling genes. Studies to validate the efficacy of selected candidate COVID-19 drugs confirm that remdesivir strongly suppresses viral infection/replication. We provide a relevant platform for study of COVID-19 pathobiology and for rapid drug screening against SARS-CoV-2 and emergent respiratory pathogens.
Keywords: COVID-19; SARS-CoV-2; adult lung epithelium; alveolar type 2 cells; alveoli; drug discovery; interferon; primary in vitro model; remdesivir.
Copyright © 2021. Published by Elsevier Inc.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


Update of
-
SARS-CoV-2 infection of primary human lung epithelium for COVID-19 modeling and drug discovery.bioRxiv [Preprint]. 2020 Jun 29:2020.06.29.174623. doi: 10.1101/2020.06.29.174623. bioRxiv. 2020. Update in: Cell Rep. 2021 May 4;35(5):109055. doi: 10.1016/j.celrep.2021.109055. PMID: 32637946 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

