Effect of tissue microenvironment on fibrous capsule formation to biomaterial-coated implants
- PMID: 33905960
- PMCID: PMC8135119
- DOI: 10.1016/j.biomaterials.2021.120806
Effect of tissue microenvironment on fibrous capsule formation to biomaterial-coated implants
Abstract
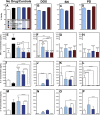
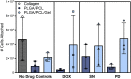
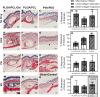
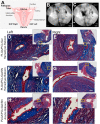
Within tissue exposed to the systemic immune system, lymphocytes and fibroblasts act against biomaterials via the development of a fibrous capsule, known as the foreign body reaction (FBR). Inspired by the natural tolerance that the uterine cavity has to foreign bodies, our study explores the role of microenvironment across classical (subcutaneous) and immune privileged (uterine) tissues in the development of the FBR. As a model biomaterial, we used electrospun fibers loaded with sclerosing agents to provoke scar tissue growth. Additionally, we integrated these materials onto an intrauterine device as a platform for intrauterine biomaterial studies. Polyester materials in vitro achieved drug release up to 10 days, greater pro-inflammatory and pro-healing cytokine expression, and the addition of gelatin enabled greater fibroblast attachment. We observed the materials that induced the greatest FBR in the mouse, had no effect when inserted at the utero-tubal junction of non-human primates. These results suggest that the FBR varies across different tissue microenvironments, and a dampened fibrotic response exists in the uterine cavity, possibly due to immune privilege. Further study of immune privileged tissue factors on biomaterials could broaden our understanding of the FBR and inform new methods for achieving biocompatibility in vivo.
Keywords: Electrospun fibers; Female reproductive tract; Foreign body reaction; Immune privilege; Intrauterine device; Sclerosing agents.
Copyright © 2021 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Dr. Jensen has received payments for consulting from Abbvie, Cooper Surgical, Bayer Healthcare, Evofem, Mayne Pharma, Merck, Sebela, and TherapeuticsMD. OHSU has received research support from Abbvie, Bayer Healthcare, Daré, Estetra SPRL, Medicines360, Merck, and Sebela. These companies and organizations may have a commercial or financial interest in the results of this research and technology. These potential conflicts of interest have been reviewed and managed by OHSU. Bob Katz is a paid consultant for Sebela Pharmaceuticals and CEO of Hybridge Medical, LLC.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

