Overview of Secondary Neurulation
- PMID: 33906344
- PMCID: PMC8128529
- DOI: 10.3340/jkns.2020.0362
Overview of Secondary Neurulation
Abstract
Secondary neurulation is a morphological process described since the second half of the 19th century; it accounts for the formation of the caudal spinal cord in mammals including humans. A similar process takes place in birds. This form of neurulation is caused by the growth of the tail bud region, the most caudal axial region of the embryo. Experimental work in different animal species leads to questioning dogmas widely disseminated in the medical literature. Thus, it is clearly established that the tail bud is not a mass of undifferentiated pluripotent cells but is made up of a juxtaposition of territories whose fate is different. The lumens of the two tubes generated by the two modes of neurulation are continuous. There seem to be multiple cavities in the human embryo, but discrepancies exist according to the authors. Finally, the tissues that generate the secondary neural tube are initially located in the most superficial layer of the embryo. These cells must undergo internalization to generate the secondary neurectoderm. A defect in internalization could lead to an open neural tube defect that contradicts the dogma that a secondary neurulation defect is closed by definition.
Keywords: Blastema; Caudal spinal cord; Cavitation; Secondary neurulation; Tail bud.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures



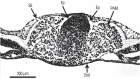








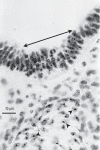






References
-
- Beck CW, Slack JM. Analysis of the developing Xenopus tail bud reveals separate phases of gene expression during determination and outgrowth. Mech Dev. 1998;72:41–52. - PubMed
-
- Bijtel JH. Über die Entwicklung des Schwanzes bei Amphibien. Wilhelm Roux Arch Entwickl Mech Org. 1931;125:448–486. - PubMed
-
- Braun M. Entwickelungsvorgänge am Schwanzende bei einigen Säugethieren mit Berücksichtigungder Verhältnisse beim Menschen. Arch f Anat u Phys, Anat Abt. 1882;6:207–241.
-
- Cambray N, Wilson V. Axial progenitors with extensive potency are localised to the mouse chordoneural hinge. Development. 2002;129:4855–4866. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

