Salt Transiently Inhibits Mitochondrial Energetics in Mononuclear Phagocytes
- PMID: 33906377
- PMCID: PMC8270232
- DOI: 10.1161/CIRCULATIONAHA.120.052788
Salt Transiently Inhibits Mitochondrial Energetics in Mononuclear Phagocytes
Erratum in
-
Correction to: Salt Transiently Inhibits Mitochondrial Energetics in Mononuclear Phagocytes.Circulation. 2021 Jul 13;144(2):e37. doi: 10.1161/CIR.0000000000001008. Epub 2021 Jul 12. Circulation. 2021. PMID: 34251896 Free PMC article. No abstract available.
Abstract
Background: Dietary high salt (HS) is a leading risk factor for mortality and morbidity. Serum sodium transiently increases postprandially but can also accumulate at sites of inflammation affecting differentiation and function of innate and adaptive immune cells. Here, we focus on how changes in extracellular sodium, mimicking alterations in the circulation and tissues, affect the early metabolic, transcriptional, and functional adaption of human and murine mononuclear phagocytes.
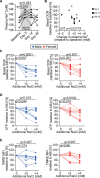
Methods: Using Seahorse technology, pulsed stable isotope-resolved metabolomics, and enzyme activity assays, we characterize the central carbon metabolism and mitochondrial function of human and murine mononuclear phagocytes under HS in vitro. HS as well as pharmacological uncoupling of the electron transport chain under normal salt is used to analyze mitochondrial function on immune cell activation and function (as determined by Escherichiacoli killing and CD4+ T cell migration capacity). In 2 independent clinical studies, we analyze the effect of a HS diet during 2 weeks (URL: http://www.clinicaltrials.gov. Unique identifier: NCT02509962) and short-term salt challenge by a single meal (URL: http://www.clinicaltrials.gov. Unique identifier: NCT04175249) on mitochondrial function of human monocytes in vivo.
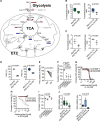
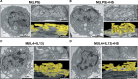
Results: Extracellular sodium was taken up into the intracellular compartment, followed by the inhibition of mitochondrial respiration in murine and human macrophages. Mechanistically, HS reduces mitochondrial membrane potential, electron transport chain complex II activity, oxygen consumption, and ATP production independently of the polarization status of macrophages. Subsequently, cell activation is altered with improved bactericidal function in HS-treated M1-like macrophages and diminished CD4+ T cell migration in HS-treated M2-like macrophages. Pharmacological uncoupling of the electron transport chain under normal salt phenocopies HS-induced transcriptional changes and bactericidal function of human and murine mononuclear phagocytes. Clinically, also in vivo, rise in plasma sodium concentration within the physiological range reversibly reduces mitochondrial function in human monocytes. In both a 14-day and single meal HS challenge, healthy volunteers displayed a plasma sodium increase of [Formula: see text] and [Formula: see text] respectively, that correlated with decreased monocytic mitochondrial oxygen consumption.
Conclusions: Our data identify the disturbance of mitochondrial respiration as the initial step by which HS mechanistically influences immune cell function. Although these functional changes might help to resolve bacterial infections, a shift toward proinflammation could accelerate inflammatory cardiovascular disease.
Keywords: bacterial killing, humans; complex II; macrophages; metabolism; mitochondrial respiration; monocytes; salt.
Figures



References
-
- Taylor J. 2013 ESH/ESC guidelines for the management of arterial hypertension. Eur Heart J. 2013;34:2108–2109 - PubMed
-
- Müller DN, Wilck N, Haase S, Kleinewietfeld M, Linker RA. Sodium in the microenvironment regulates immune responses and tissue homeostasis. Nat Rev Immunol. 2019;19:243–254. doi: 10.1038/s41577-018-0113-4 - PubMed
-
- Mozaffarian D, Fahimi S, Singh GM, Micha R, Khatibzadeh S, Engell RE, Lim S, Danaei G, Ezzati M, Powles J; Global Burden of Diseases Nutrition and Chronic Diseases Expert Group. Global sodium consumption and death from cardiovascular causes. N Engl J Med. 2014;371:624–634. doi: 10.1056/NEJMoa1304127 - PubMed
-
- Suckling RJ, He FJ, Markandu ND, MacGregor GA. Dietary salt influences postprandial plasma sodium concentration and systolic blood pressure. Kidney Int. 2012;81:407–411. doi: 10.1038/ki.2011.369 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

