Effect of Apabetalone on Cardiovascular Events in Diabetes, CKD, and Recent Acute Coronary Syndrome: Results from the BETonMACE Randomized Controlled Trial
- PMID: 33906908
- PMCID: PMC8259488
- DOI: 10.2215/CJN.16751020
Effect of Apabetalone on Cardiovascular Events in Diabetes, CKD, and Recent Acute Coronary Syndrome: Results from the BETonMACE Randomized Controlled Trial
Abstract
Background and objectives: CKD and type 2 diabetes mellitus interact to increase the risk of major adverse cardiovascular events (i.e., cardiovascular death, nonfatal myocardial infarction, or stroke) and congestive heart failure. A maladaptive epigenetic response may be a cardiovascular risk driver and amenable to modification with apabetalone, a selective modulator of the bromodomain and extraterminal domain transcription system. We examined this question in a prespecified analysis of BETonMACE, a phase 3 trial.
Design, setting, participants, & measurements: BETonMACE was an event-driven, randomized, double-blind, placebo-controlled trial comparing effects of apabetalone versus placebo on major adverse cardiovascular events and heart failure hospitalizations in 2425 participants with type 2 diabetes and a recent acute coronary syndrome, including 288 participants with CKD with eGFR <60 ml/min per 1.73 m2 at baseline. The primary end point in BETonMACE was the time to the first major adverse cardiovascular event, with a secondary end point of time to hospitalization for heart failure.
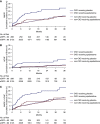
Results: Median follow-up was 27 months (interquartile range, 20-32 months). In participants with CKD, apabetalone compared with placebo was associated with fewer major adverse cardiovascular events (13 events in 124 patients [11%] versus 35 events in 164 patients [21%]; hazard ratio, 0.50; 95% confidence interval, 0.26 to 0.96) and fewer heart failure-related hospitalizations (three hospitalizations in 124 patients [3%] versus 14 hospitalizations in 164 patients [9%]; hazard ratio, 0.48; 95% confidence interval, 0.26 to 0.86). In the non-CKD group, the corresponding hazard ratio values were 0.96 (95% confidence interval, 0.74 to 1.24) for major adverse cardiovascular events, and 0.76 (95% confidence interval, 0.46 to 1.27) for heart failure-related hospitalization. Interaction of CKD on treatment effect was P=0.03 for major adverse cardiovascular events, and P=0.12 for heart failure-related hospitalization. Participants with CKD showed similar numbers of adverse events, regardless of randomization to apabetalone or placebo (119 [73%] versus 88 [71%] patients), and there were fewer serious adverse events (29% versus 43%; P=0.02) in the apabetalone group.
Conclusions: Apabetalone may reduce the incidence of major adverse cardiovascular events in patients with CKD and type 2 diabetes who have a high burden of cardiovascular disease.
Keywords: CKD stage 5; alkaline phosphatase; apabetalone; cardiovascular; chronic kidney disease; diabetes; diabetes mellitus; epigenetic pharmacotherapy; major adverse cardiovascular events.
Copyright © 2021 by the American Society of Nephrology.
Figures


Comment in
-
Novel Therapeutic Options for Cardiovascular Disease with CKD.Clin J Am Soc Nephrol. 2021 May 8;16(5):682-684. doi: 10.2215/CJN.03270321. Epub 2021 Apr 27. Clin J Am Soc Nephrol. 2021. PMID: 33906909 Free PMC article. No abstract available.
References
-
- United States Renal Data System : 2020 USRDS Annual Data Report: Epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2020. Available at: https://adr.usrds.org/2020. Accessed February 18, 2021
-
- Go AS, Chertow GM, Fan D, McCulloch CE, Hsu C-Y: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization [published correction appears in N Engl J Med 18: 4, 2008]. N Engl J Med 351: 1296–1305, 2004. - PubMed
-
- Haarhaus M, Brandenburg V, Kalantar-Zadeh K, Stenvinkel P, Magnusson P: Alkaline phosphatase: A novel treatment target for cardiovascular disease in CKD. Nat Rev Nephrol 13: 429–442, 2017. - PubMed
-
- de Zeeuw D, Anzalone DA, Cain VA, Cressman MD, Heerspink HJ, Molitoris BA, Monyak JT, Parving HH, Remuzzi G, Sowers JR, Vidt DG: Renal effects of atorvastatin and rosuvastatin in patients with diabetes who have progressive renal disease (PLANET I): A randomised clinical trial. Lancet Diabetes Endocrinol 3: 181–190, 2015. - PubMed
-
- Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu PL, de Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW; CREDENCE Trial Investigators: Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 380: 2295–2306, 2019. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

