Analysis of the Long-Term Impact on Cellular Immunity in COVID-19-Recovered Individuals Reveals a Profound NKT Cell Impairment
- PMID: 33906918
- PMCID: PMC8092197
- DOI: 10.1128/mBio.00085-21
Analysis of the Long-Term Impact on Cellular Immunity in COVID-19-Recovered Individuals Reveals a Profound NKT Cell Impairment
Abstract
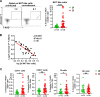
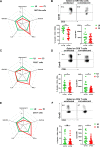
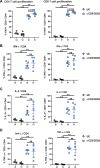
The coronavirus disease 2019 (COVID-19) pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) affected over 120 million people and killed over 2.7 million individuals by March 2021. While acute and intermediate interactions between SARS-CoV-2 and the immune system have been studied extensively, long-term impacts on the cellular immune system remain to be analyzed. Here, we comprehensively characterized immunological changes in peripheral blood mononuclear cells in 49 COVID-19-convalescent individuals (CI) in comparison to 27 matched SARS-CoV-2-unexposed individuals (UI). Despite recovery from the disease for more than 2 months, CI showed significant decreases in frequencies of invariant NKT and NKT-like cells compared to UI. Concomitant with the decrease in NKT-like cells, an increase in the percentage of annexin V and 7-aminoactinomycin D (7-AAD) double-positive NKT-like cells was detected, suggesting that the reduction in NKT-like cells results from cell death months after recovery. Significant increases in regulatory T cell frequencies and TIM-3 expression on CD4 and CD8 T cells were also observed in CI, while the cytotoxic potential of T cells and NKT-like cells, defined by granzyme B (GzmB) expression, was significantly diminished. However, both CD4 and CD8 T cells of CI showed increased Ki67 expression and were fully able to proliferate and produce effector cytokines upon T cell receptor (TCR) stimulation. Collectively, we provide a comprehensive characterization of immune signatures in patients recovering from SARS-CoV-2 infection, suggesting that the cellular immune system of COVID-19 patients is still under a sustained influence even months after the recovery from disease.IMPORTANCE Wuhan was the very first city hit by SARS-CoV-2. Accordingly, the patients who experienced the longest phase of convalescence following COVID-19 reside here. This enabled us to investigate the "immunological scar" left by SARS-CoV-2 on cellular immunity after recovery from the disease. In this study, we characterized the long-term impact of SARS-CoV-2 infection on the immune system and provide a comprehensive picture of cellular immunity of a convalescent COVID-19 patient cohort with the longest recovery time. We revealed that the cellular immune system of COVID-19 patients is still under a sustained influence even months after the recovery from disease; in particular, a profound NKT cell impairment was found in the convalescent phase of COVID-19.
Keywords: COVID-19; NKT cell; SARS-CoV-2; cellular immunity.
Copyright © 2021 Liu et al.
Figures


References
-
- Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS, China Medical Treatment Expert Group for Covid-19. 2020. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382:1708–1720. 10.1056/NEJMoa2002032. - DOI - PMC - PubMed
-
- Puelles VG, Lutgehetmann M, Lindenmeyer MT, Sperhake JP, Wong MN, Allweiss L, Chilla S, Heinemann A, Wanner N, Liu S, Braun F, Lu S, Pfefferle S, Schroder AS, Edler C, Gross O, Glatzel M, Wichmann D, Wiech T, Kluge S, Pueschel K, Aepfelbacher M, Huber TB. 2020. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med 383:590–592. 10.1056/NEJMc2011400. - DOI - PMC - PubMed
-
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. 2020. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395:497–506. 10.1016/S0140-6736(20)30183-5. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
