ColdZyme Maintains Integrity in SARS-CoV-2-Infected Airway Epithelia
- PMID: 33906927
- PMCID: PMC8092264
- DOI: 10.1128/mBio.00904-21
ColdZyme Maintains Integrity in SARS-CoV-2-Infected Airway Epithelia
Abstract
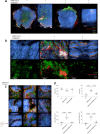
SARS-CoV-2 infection causing the COVID-19 pandemic calls for immediate interventions to avoid viral transmission, disease progression, and subsequent excessive inflammation and tissue destruction. Primary normal human bronchial epithelial cells are among the first targets of SARS-CoV-2 infection. Here, we show that ColdZyme medical device mouth spray efficiently protected against virus entry, excessive inflammation, and tissue damage. Applying ColdZyme to fully differentiated, polarized human epithelium cultured at an air-liquid interphase (ALI) completely blocked binding of SARS-CoV-2 and increased local complement activation mediated by the virus as well as productive infection of the tissue model. While SARS-CoV-2 infection resulted in exaggerated intracellular complement activation immediately following infection and a drop in transepithelial resistance, these parameters were bypassed by single pretreatment of the tissues with ColdZyme mouth spray. Crucially, our study highlights the importance of testing already evaluated and safe drugs such as ColdZyme mouth spray for maintaining epithelial integrity and hindering SARS-CoV-2 entry within standardized three-dimensional (3D) in vitro models mimicking the in vivo human airway epithelium.IMPORTANCE Although our understanding of COVID-19 continuously progresses, essential questions regarding prophylaxis and treatment remain open. A hallmark of severe SARS-CoV-2 infection is a hitherto-undescribed mechanism leading to excessive inflammation and tissue destruction associated with enhanced pathogenicity and mortality. To tackle the problem at the source, transfer of SARS-CoV-2, subsequent binding, infection, and inflammatory responses have to be avoided. In this study, we used fully differentiated, mucus-producing, and ciliated human airway epithelial cultures to test the efficacy of ColdZyme medical device mouth spray in terms of protection from SARS-CoV-2 infection. Importantly, we found that pretreatment of the in vitro airway cultures using ColdZyme mouth spray resulted in significantly shielding the epithelial integrity, hindering virus binding and infection, and blocking excessive intrinsic complement activation within the airway cultures. Our in vitro data suggest that ColdZyme mouth spray may have an impact in prevention of COVID-19.
Keywords: ColdZyme; SARS-CoV-2; airway epithelia; anaphylatoxins; antiviral response.
Copyright © 2021 Posch et al.
Figures

References
-
- Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu N-H, Nitsche A, Müller MA, Drosten C, Pöhlmann S. 2020. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181:271–280.e278. doi:10.1016/j.cell.2020.02.052. - DOI - PMC - PubMed
-
- Ziegler CGK, Allon SJ, Nyquist SK, Mbano IM, Miao VN, Tzouanas CN, Cao Y, Yousif AS, Bals J, Hauser BM, Feldman J, Muus C, Wadsworth MH, Kazer SW, Hughes TK, Doran B, Gatter GJ, Vukovic M, Taliaferro F, Mead BE, Guo Z, Wang JP, Gras D, Plaisant M, Ansari M, Angelidis I, Adler H, Sucre JMS, Taylor CJ, Lin B, Waghray A, Mitsialis V, Dwyer DF, Buchheit KM, Boyce JA, Barrett NA, Laidlaw TM, Carroll SL, Colonna L, Tkachev V, Peterson CW, Yu A, Zheng HB, Gideon HP, Winchell CG, Lin PL, Bingle CD, Snapper SB, Kropski JA, Theis FJ, HCA Lung Biological Network, et al. . 2020. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell 181:1016–1035.e19. doi:10.1016/j.cell.2020.04.035. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

