Insulin-Dependent Maturation of Newly Generated Olfactory Sensory Neurons after Injury
- PMID: 33906971
- PMCID: PMC8143024
- DOI: 10.1523/ENEURO.0168-21.2021
Insulin-Dependent Maturation of Newly Generated Olfactory Sensory Neurons after Injury
Abstract
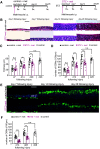
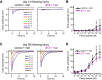
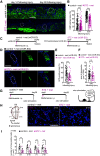
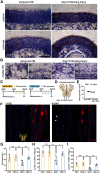
Loss of olfactory sensory neurons (OSNs) after injury to the olfactory epithelium (OE) triggers the generation of OSNs that are incorporated into olfactory circuits to restore olfactory sensory perception. This study addresses how insulin receptor-mediated signaling affects the functional recovery of OSNs after OE injury. Insulin levels were reduced in mice by ablating the pancreatic β cells via streptozotocin (STZ) injections. These STZ-induced diabetic and control mice were then intraperitoneally injected with the olfactotoxic drug methimazole to selectively ablate OSNs. The OE of diabetic and control mice regenerated similarly until day 14 after injury. Thereafter, the OE of diabetic mice contained fewer mature and more apoptotic OSNs than control mice. Functionally, diabetic mice showed reduced electro-olfactogram (EOG) responses and their olfactory bulbs (OBs) had fewer c-Fos-active cells following odor stimulation, as well as performed worse in an odor-guided task compared with control mice. Insulin administered intranasally during days 8-13 after injury was sufficient to rescue recovery of OSNs in diabetic mice compared with control levels, while insulin administration between days 1 and 6 did not. During this critical time window on days 8-13 after injury, insulin receptors are highly expressed and intranasal application of an insulin receptor antagonist inhibits regeneration. Furthermore, an insulin-enriched environment could facilitate regeneration even in non-diabetic mice. These results indicate that insulin facilitates the regeneration of OSNs after injury and suggest a critical stage during recovery (8-13 d after injury) during which the maturation of newly generated OSNs is highly dependent on and promoted by insulin.
Keywords: diabetes mellitus; electro-olfactogram; insulin; olfactory sensory neurons; regeneration; streptozotocin.
Copyright © 2021 Kuboki et al.
Figures







References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
