Early loss of Scribble affects cortical development, interhemispheric connectivity and psychomotor activity
- PMID: 33907211
- PMCID: PMC8079449
- DOI: 10.1038/s41598-021-88147-1
Early loss of Scribble affects cortical development, interhemispheric connectivity and psychomotor activity
Abstract
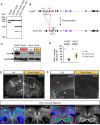
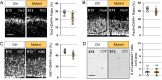
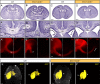
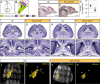
Neurodevelopmental disorders arise from combined defects in processes including cell proliferation, differentiation, migration and commissure formation. The evolutionarily conserved tumor-suppressor protein Scribble (Scrib) serves as a nexus to transduce signals for the establishment of apicobasal and planar cell polarity during these processes. Human SCRIB gene mutations are associated with neural tube defects and this gene is located in the minimal critical region deleted in the rare Verheij syndrome. In this study, we generated brain-specific conditional cKO mouse mutants and assessed the impact of the Scrib deletion on brain morphogenesis and behavior. We showed that embryonic deletion of Scrib in the telencephalon leads to cortical thickness reduction (microcephaly) and partial corpus callosum and hippocampal commissure agenesis. We correlated these phenotypes with a disruption in various developmental mechanisms of corticogenesis including neurogenesis, neuronal migration and axonal connectivity. Finally, we show that Scrib cKO mice have psychomotor deficits such as locomotor activity impairment and memory alterations. Altogether, our results show that Scrib is essential for early brain development due to its role in several developmental cellular mechanisms that could underlie some of the deficits observed in complex neurodevelopmental pathologies.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

