Cytotoxic, analgesic and anti-inflammatory activity of colchicine and its C-10 sulfur containing derivatives
- PMID: 33907227
- PMCID: PMC8079405
- DOI: 10.1038/s41598-021-88260-1
Cytotoxic, analgesic and anti-inflammatory activity of colchicine and its C-10 sulfur containing derivatives
Abstract
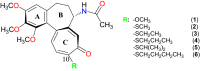
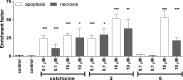
10-Alkylthiocolchicines have been obtained and characterized by spectroscopic methods and their biological activities as: cytotoxic, anti-inflammatory and analgesic activities have been tested. Cytotoxic activity against SKOV-3 ovarian cell line for 10-alkylthiocolchicine analogues was reported and tested compounds showed to be more active than commonly used doxorubicin. Some of tested C-10 alkylthiolated colchicines have been found to exhibit cytotoxicity at levels comparable to that of the natural product-colchicine. 10-Methylthiocolchicine has IC50 = 8 nM and 10-ethylthiocolchicine has IC50 = 47 nM in comparison to colchicine IC50 = 37 nM. Moreover for 10-alkylthioderivatives apoptosis test, cyclin B1 and cell cycle tests were performed. 10-n-Butylthiocolchicine was tested for anti-inflammatory and analgesic activities it showed to produce analgesic rather than anti-inflammatory effect.
Conflict of interest statement
The authors declare no competing interests
Figures






Similar articles
-
Synthesis, anticancer activity and molecular docking studies of N-deacetylthiocolchicine and 4-iodo-N-deacetylthiocolchicine derivatives.Bioorg Med Chem. 2021 Feb 15;32:116014. doi: 10.1016/j.bmc.2021.116014. Epub 2021 Jan 11. Bioorg Med Chem. 2021. PMID: 33465696
-
Carbamate derivatives of colchicine show potent activity towards primary acute lymphoblastic leukemia and primary breast cancer cells-in vitro and ex vivo study.J Biochem Mol Toxicol. 2020 Jun;34(6):e22487. doi: 10.1002/jbt.22487. Epub 2020 Mar 5. J Biochem Mol Toxicol. 2020. PMID: 32141170
-
A novel microtubule depolymerizing colchicine analogue triggers apoptosis and autophagy in HCT-116 colon cancer cells.Cell Biochem Funct. 2016 Mar;34(2):69-81. doi: 10.1002/cbf.3166. Cell Biochem Funct. 2016. PMID: 26919061
-
Potential anticancer role of colchicine-based derivatives: an overview.Anticancer Drugs. 2017 Mar;28(3):250-262. doi: 10.1097/CAD.0000000000000464. Anticancer Drugs. 2017. PMID: 28030380 Review.
-
Recent progress in structure-activity relationship studies on the anticancer drug colchicine and its analogues.Yao Xue Xue Bao. 2002 Oct;37(10):821-7. Yao Xue Xue Bao. 2002. PMID: 12567870 Review. No abstract available.
Cited by
-
Phragmanthera austroarabica A.G.Mill. and J.A.Nyberg Triggers Apoptosis in MDA-MB-231 Cells In Vitro and In Vivo Assays: Simultaneous Determination of Selected Constituents.Metabolites. 2022 Sep 29;12(10):921. doi: 10.3390/metabo12100921. Metabolites. 2022. PMID: 36295823 Free PMC article.
-
Regioselective approach to colchiceine tropolone ring functionalization at C(9) and C(10) yielding new anticancer hybrid derivatives containing heterocyclic structural motifs.J Enzyme Inhib Med Chem. 2022 Dec;37(1):597-605. doi: 10.1080/14756366.2022.2028782. J Enzyme Inhib Med Chem. 2022. PMID: 35067138 Free PMC article.
-
Bottlebrush Polyethylene Glycol Nanocarriers Translocate across Human Airway Epithelium via Molecular Architecture-Enhanced Endocytosis.ACS Nano. 2024 Jul 9;18(27):17586-17599. doi: 10.1021/acsnano.4c01983. Epub 2024 Jun 27. ACS Nano. 2024. PMID: 38932624 Free PMC article.
-
Discovery and development of botanical natural products and their analogues as therapeutics for ovarian cancer.Nat Prod Rep. 2023 Jul 19;40(7):1250-1270. doi: 10.1039/d2np00091a. Nat Prod Rep. 2023. PMID: 37387219 Free PMC article. Review.
References
-
- Cutler, S.J., Cutler, H.G. Biologically Active Natural Products. Pharmaceuticals. 1st edn, (eds Cutler, S. J., Cutler H. G. CRC Press Taylor & Francis Books, 2000).
-
- Bhat SV, Nagasampagi BA, Sivakumar M. Chemistry of Natural Products. Springer; 2005.
-
- Kurek J, Boczoń W, Murias M, Myszkowski K, et al. Synthesis of sulfur containing colchicine derivatives and their biological evaluation as cytotoxic agents. Lett Drug Des. Discov. 2014;11(3):279–289. doi: 10.2174/15701808113106660086. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

