Altered neural oscillations and behavior in a genetic mouse model of NMDA receptor hypofunction
- PMID: 33907230
- PMCID: PMC8079688
- DOI: 10.1038/s41598-021-88428-9
Altered neural oscillations and behavior in a genetic mouse model of NMDA receptor hypofunction
Abstract
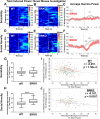
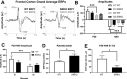
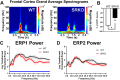
Abnormalities in electroencephalographic (EEG) biomarkers occur in patients with schizophrenia and those clinically at high risk for transition to psychosis and are associated with cognitive impairment. Converging evidence suggests N-methyl-D-aspartate receptor (NMDAR) hypofunction plays a central role in the pathophysiology of schizophrenia and likely contributes to biomarker impairments. Thus, characterizing these biomarkers is of significant interest for early diagnosis of schizophrenia and development of novel treatments. We utilized in vivo EEG recordings and behavioral analyses to perform a battery of electrophysiological biomarkers in an established model of chronic NMDAR hypofunction, serine racemase knockout (SRKO) mice, and their wild-type littermates. SRKO mice displayed impairments in investigation-elicited gamma power that corresponded with reduced short-term social recognition and enhanced background (pre-investigation) gamma activity. Additionally, SRKO mice exhibited sensory gating impairments in both evoked-gamma power and event-related potential amplitude. However, other biomarkers including the auditory steady-state response, sleep spindles, and state-specific power spectral density were generally neurotypical. In conclusion, SRKO mice demonstrate how chronic NMDAR hypofunction contributes to deficits in certain translationally-relevant EEG biomarkers altered in schizophrenia. Importantly, our gamma band findings suggest an aberrant signal-to-noise ratio impairing cognition that occurs with NMDAR hypofunction, potentially tied to impaired task-dependent alteration in functional connectivity.
Conflict of interest statement
All authors except JTC and DTB declare no competing financial interests in relation to the work described. JTC reports holding a patent on D-serine to treat serious mental disorder that is owned by Massachusetts General Hospital but could yield royalties, and a patent on an AI-based EEG method to predict psychotropic drug response. DTB served as a consultant for LifeSci Capital and received research support from Takeda Pharmaceuticals.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

