Minor to Moderate Side Effects of Pfizer-BioNTech COVID-19 Vaccine Among Saudi Residents: A Retrospective Cross-Sectional Study
- PMID: 33907443
- PMCID: PMC8068468
- DOI: 10.2147/IJGM.S310497
Minor to Moderate Side Effects of Pfizer-BioNTech COVID-19 Vaccine Among Saudi Residents: A Retrospective Cross-Sectional Study
Abstract
Background: The Pfizer-BioNTech COVID-19 vaccine has recently received emergency approval from the US FDA. The mRNA technology was used to manufacture the Pfizer vaccine; however, as a pioneering technology that has never been used in the manufacture of vaccines, many people have concerns about the vaccine's side effects. Thus, the current study aimed to track the short-term side effects of the vaccine.
Methods: The information in this study was gathered by a Google Form-questionnaire (online survey). The results included the responses of 455 individuals, all of whom are Saudi Arabia inhabitants. Adverse effects of the vaccine were reported after the first and the second doses.
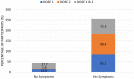
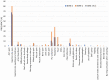
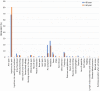
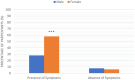
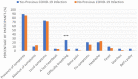
Results: The most common symptoms were injection site pain, headaches, flu-like symptoms, fever, and tiredness. Less common side effects were a fast heartbeat, whole body aches, difficulty breathing, joint pain, chills, and drowsiness. Rare side effects include Bell's palsy and lymph nodes swelling and tenderness. Flu-like symptoms were more common among those under 60 years of age, while injection site pain was more frequent among recipients who were 60 years and older. The study revealed a significant increase in the number of females who suffered from the vaccine side effects compared to males. Difficulty of breathing was more reported among recipients who had been previously infected with the coronavirus compared to those who had not been previously infected.
Conclusion: Most of the side effects reported in this study were consistent with Pfizer's fact sheet for recipients and caregivers. Further studies are required to determine the long-term side effects.
Keywords: Pfizer-BioNTech COVID-19 vaccine; flu-like symptoms; hypersensitivity; injection site pain; online survey; side effects.
© 2021 El-Shitany et al.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Comment in
-
Acute exacerbation of idiopathic pulmonary fibrosis after SARS-CoV-2 vaccination.Eur Respir J. 2022 Mar 10;59(3):2102806. doi: 10.1183/13993003.02806-2021. Print 2022 Mar. Eur Respir J. 2022. PMID: 35144990 Free PMC article.
References
-
- World Health Organization. Coronavirus disease (COVID-19) situation report-127. World Health Organisation (WHO); 2020:1–17. Available from: https://apps.who.int/iris/handle/10665/332232. Accessed February20, 2021.
-
- World Health Organisation. Draft landscape and tracker of COVID-19 candidate vaccines. World Health Organisation (WHO); 2020. Available from: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-cand.... Accessed February20, 2021.
-
- FDA Briefing Document. Pfizer-BioNTech COVID-19 vaccine. U.S. Food and Drug Administration; 2020:1–53. Available from: https://www.fda.gov/media/144245/download. Accessed February11, 2021.
-
- FDA Briefing Document. Moderna COVID-19 Vaccine. U.S. Food and Drug Administration; 2020:1–54. Available from: https://www.fda.gov/media/144434/download. Accessed February11, 2021.
LinkOut - more resources
Full Text Sources
Other Literature Sources

