Positive regulation of TNFR1 signaling via SH3 recognition motif
- PMID: 33907493
- PMCID: PMC8068768
- DOI: 10.3906/biy-2010-28
Positive regulation of TNFR1 signaling via SH3 recognition motif
Abstract
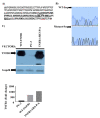
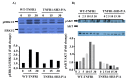
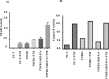
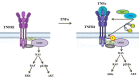
TNF is a pleiotropic cytokine and shows its biological function by binding to its receptors called TNFR1 and TNFR2. While TNFR1 induces apoptosis by activation of caspase-8 via the "death domain", it also activates IKKα/β, MKK3/6, MKK4/7 by activation of TAK1. Although the TNFR1 signaling pathway is known by in large, it is not known how AKT and MAPKs p38, ERK1/2, and JNK1/2 are activated. The presence of a proline-rich PPAP region, (P448PAP451, a binding site for the SH3 domain-containing proteins) very close to the C-terminus promoted us to determine whether this region has any role in the TNFR1 signal transduction. To test this, the codons of P448 and P451 were changed to that of Alanin, GCG, via site-directed mutagenesis, and this plasmid was named as TNFR1-SH3-P/A. Subsequently, ectopically expressed the wild type TNFR1 and TNFR1-SH3-P/A in 293T cells and determined the levels of TNF-α-mediated phosphorylations of ERK, p38, JNK and AKT, NF-kB, and caspase-8 activation. While ectopic expression of our mutant diminished TNFα-mediated phosphorylations of p38, JNK, ERK and AKT, it increased NF-kB, and caspase-8 activations. In conclusion, TNFα-mediated ERK, AKT, JNK, p38 activations are affected by TNFR1 SH3 domain modifications.
Keywords: AKT; ERK; Grb2; TNFR1; TNF-α.
Copyright © 2021 The Author(s).
Conflict of interest statement
CONFLICT OF INTEREST: none declared
Figures

Similar articles
-
TNFR1 signaling is positively regulated by Jak-2 and c-Src via tyrosine phosphorylation.Turk J Biol. 2023 Nov 6;48(1):1-12. doi: 10.55730/1300-0152.2677. eCollection 2024. Turk J Biol. 2023. PMID: 38665776 Free PMC article.
-
Negative Regulation of TNFR1 Signaling Via PKA-Mediated Phosphorylation of TNFR1.J Interferon Cytokine Res. 2020 May;40(5):225-235. doi: 10.1089/jir.2019.0128. Epub 2020 Mar 10. J Interferon Cytokine Res. 2020. PMID: 32159413
-
Progranulin promotes osteogenic differentiation of human periodontal ligament stem cells via tumor necrosis factor receptors to inhibit TNF-α sensitized NF-kB and activate ERK/JNK signaling.J Periodontal Res. 2020 Jun;55(3):363-373. doi: 10.1111/jre.12720. Epub 2019 Dec 19. J Periodontal Res. 2020. PMID: 31854464
-
TRAF2 multitasking in TNF receptor-induced signaling to NF-κB, MAP kinases and cell death.Biochem Pharmacol. 2016 Sep 15;116:1-10. doi: 10.1016/j.bcp.2016.03.009. Epub 2016 Mar 16. Biochem Pharmacol. 2016. PMID: 26993379 Review.
-
Structural determinants of DISC function: new insights into death receptor-mediated apoptosis signalling.Pharmacol Ther. 2013 Nov;140(2):186-99. doi: 10.1016/j.pharmthera.2013.06.009. Epub 2013 Jul 8. Pharmacol Ther. 2013. PMID: 23845861 Review.
Cited by
-
TNFR1 signaling is positively regulated by Jak-2 and c-Src via tyrosine phosphorylation.Turk J Biol. 2023 Nov 6;48(1):1-12. doi: 10.55730/1300-0152.2677. eCollection 2024. Turk J Biol. 2023. PMID: 38665776 Free PMC article.
References
-
- Bertr MJ Milutinovic S Dickson KM Ho WC Boudreault A cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Molecular Cell. 2008;6:689–700. - PubMed
-
- Boone E Vandevoorde V Wilde GD Haegeman G Activation of p42/p44 mitogen-activated protein kinases (MAPK) and p38 MAPK by tumor necrosis factor (TNF) is mediated through the death domain of the 55-kDa TNF receptor. FEBS Letters. 1998;2:275–280. - PubMed
-
- Ea CK Deng L Xia ZP Pineda G Chen ZJ Activation of IKK by TNFalpha requires site-specific ubiquitination of RIP1 and polyubiquitin binding by NEMO. Molecular Cell. 2006;2:245–257. - PubMed
-
- Gerlach B Cordier SM Schmukle AC Emmerich CH Rieser E Linear ubiquitination prevents inflammation and regulates immune signalling. Nature. 2011;7340:591–596. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
