Development and application of therapeutic antibodies against COVID-19
- PMID: 33907512
- PMCID: PMC8071770
- DOI: 10.7150/ijbs.59149
Development and application of therapeutic antibodies against COVID-19
Abstract
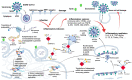
The pandemic of Coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome 2 coronavirus (SARS-CoV-2) continues to be a global health crisis. Fundamental studies at genome, transcriptome, proteome, and interactome levels have revealed many viral and host targets for therapeutic interventions. Hundreds of antibodies for treating COVID-19 have been developed at preclinical and clinical stages in the format of polyclonal antibodies, monoclonal antibodies, and cocktail antibodies. Four products, i.e., convalescent plasma, bamlanivimab, REGN-Cov2, and the cocktail of bamlanivimab and etesevimab have been authorized by the U.S. Food and Drug Administration (FDA) for emergency use. Hundreds of relevant clinical trials are ongoing worldwide. Therapeutic antibody therapies have been a very active and crucial part of COVID-19 treatment. In this review, we focus on the progress of therapeutic COVID-19 antibody development and application, discuss corresponding problems and challenges, suggesting new strategies and solutions.
Keywords: COVID-19; SARS-CoV-2; antibody cocktail; convalescent plasma; monoclonal antibody; therapeutic antibody.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
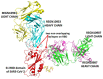
References
-
- WHO. WHO Coronavirus Disease (COVID-19) Dashboard [Internet] Available from: https://covid19.who.int.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

