Inhibition of aquaporins as a potential adjunct to breast cancer cryotherapy
- PMID: 33907568
- PMCID: PMC8063341
- DOI: 10.3892/ol.2021.12719
Inhibition of aquaporins as a potential adjunct to breast cancer cryotherapy
Abstract
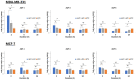
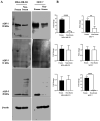
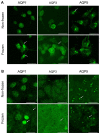
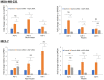
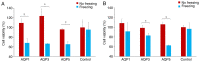
Cryoablation is an emerging type of treatment for cancer. The sensitization of tumors using cryosensitizing agents prior to treatment enhances ablation efficiency and may improve clinical outcomes. Water efflux, which is regulated by aquaporin channels, contributes to cancer cell damage achieved through cryoablation. An increase in aquaporin (AQP) 3 is cryoprotective, whereas its inhibition augments cryodamage. The present study aimed to investigate aquaporin (AQP1, AQP3 and AQP5) gene expression and cellular localization in response to cryoinjury. Cultured breast cancer cells (MDA-MB-231 and MCF-7) were exposed to freezing to induce cryoinjury. RNA and protein extracts were then analyzed using reverse transcription-quantitative PCR and western blotting, respectively. Localization of aquaporins was studied using immunocytochemistry. Additionally, cells were transfected with small interfering RNA to silence aquaporin gene expression and cell viability was assessed using the Sulforhodamine B assay. Cryoinjury did not influence gene expression of AQPs, except for a 4-fold increase of AQP1 expression in MDA-MD-231 cells. There were no clear differences in AQP protein expression for either cell lines upon exposure to frozen and non-frozen temperatures, with the exception of fainter AQP5 bands for non-frozen MCF-7 cells. The exposure of cancer cells to freezing temperatures altered the localization of AQP1 and AQP3 proteins in both MCF-7 and MDA-MD-231 cells. The silencing of AQP1, AQP3 and AQP5 exacerbated MDA-MD-231 cell damage associated with freezing compared with control siRNA. This was also observed with AQP3 and AQP5 silencing in MCF-7 cells. Inhibition of aquaporins may potentially enhance cryoinjury. This cryosensitizing process may be used as an adjunct to breast cancer cryotherapy, especially in the border area targeted by cryoablation where freezing temperatures are not cold enough to induce cellular damage.
Keywords: aquaporins; breast cancer; cryoinjury; cryosensitization; cryotherapy.
Copyright: © Alkhalifa et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Global Burden of Disease Cancer Collaboration. Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, Dicker DJ, Chimed-Orchir O, Dandona R, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: A systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3:524–548. doi: 10.1001/jamaoncol.2016.5688. - DOI - PMC - PubMed
-
- PDQ Adult Treatment Editorial Board, corp-author. PDQ Cancer Information Summaries. National Cancer Institute (USA); Bethesda, MD: 2002. Breast cancer treatment (Adult) (PDQ®): Patient version.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
