Non-toxic sulfur inhibits LPS-induced inflammation by regulating TLR-4 and JAK2/STAT3 through IL-6 signaling
- PMID: 33907855
- PMCID: PMC8127030
- DOI: 10.3892/mmr.2021.12124
Non-toxic sulfur inhibits LPS-induced inflammation by regulating TLR-4 and JAK2/STAT3 through IL-6 signaling
Abstract
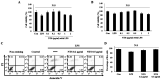
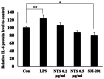
Janus kinase 2 (JAK2) and STAT3 signaling is considered a major pathway in lipopolysaccharide (LPS)‑induced inflammation. Toll‑like receptor 4 (TLR‑4) is an inflammatory response receptor that activates JAK2 during inflammation. STAT3 is a transcription factor for the pro‑inflammatory cytokine IL‑6 in inflammation. Sulfur is an essential element in the amino acids and is required for growth and development. Non‑toxic sulfur (NTS) can be used in livestock feeds as it lacks toxicity. The present study aimed to inhibit LPS‑induced inflammation in C2C12 myoblasts using NTS by regulating TLR‑4 and JAK2/STAT3 signaling via the modulation of IL‑6. The 3‑(4,5‑dimethylthiazol‑2‑yl)‑2,5‑diphenyltetrazolium bromide assay was conducted to analyze cell viability and reverse transcription polymerase chain reaction and western blotting performed to measure mRNA and protein expression levels. Chromatin immunoprecipitation and enzyme‑linked immunosorbent assays were used to determine the binding activity of proteins. The results indicated that NTS demonstrated a protective effect against LPS‑induced cell death and inhibited LPS‑induced expression of TLR‑4, JAK2, STAT3 and IL‑6. In addition, NTS inhibited the expression of nuclear phosphorylated‑STAT3 and its binding to the IL‑6 promoter. Therefore, NTS may be a potential candidate drug for the treatment of inflammation.
Keywords: IL-6; Janus kinase 2; STAT3; Toll-like receptor 4; non-toxic sulfur.
Conflict of interest statement
Hyoung Do Kim is affiliated with Nara Bio Co., Ltd., which provided funding for this study and supplied non-toxic sulfur. The remaining authors declare that they have no competing interests.
Figures




References
-
- Heinbockel L, Weindl G, Martinez-de-Tejada G, Correa W, Sanchez-Gomez S, Bárcena-Varela S, Goldmann T, Garidel P, Gutsmann T, Brandenburg K. Inhibition of lipopolysaccharide- and lipoprotein-induced inflammation by antitoxin peptide Pep19-2.5. Front Immunol. 2018;9:1704. doi: 10.3389/fimmu.2018.01704. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

