Real-time monitoring of crystallization from solution by using an interdigitated array electrode sensor
- PMID: 33908438
- PMCID: PMC8168339
- DOI: 10.1039/d0nh00685h
Real-time monitoring of crystallization from solution by using an interdigitated array electrode sensor
Abstract
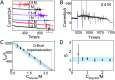
Monitoring crystallization events in real-time is challenging but crucial for understanding the molecular dynamics associated with nucleation and crystal growth, some of nature's most ubiquitous phenomena. Recent observations have suggested that the traditional nucleation model, which describes the nucleus having already the final crystal structure, may not be valid. It appears that the molecular assembly can range during nucleation from crystalline to partially ordered to totally amorphous phases, and can change its structure during the crystallization process. Therefore, it is of critical importance to develop methods that are able to provide real-time monitoring of the molecular interactions with high temporal resolution. Here, we demonstrate that a simple and scalable approach based on interdigitated electrode array sensors (IESs) is able to provide insights on the dynamics of the crystallization process with a temporal resolution of 15 ms.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Machguth H. MacFerrin M. van As D. Box J. E. Charalampidis C. Colgan W. Fausto R. S. Meijer H. A. J. Mosley-Thompson E. van de Wal R. S. W. Nat. Clim. Change. 2016;6:390–393.
-
- Weiner S. Addadi L. Annu. Rev. Mater. Res. 2011;41:21–40.
-
- Halter W. E. Williams-Jones A. E. Kontak D. J. Chem. Geol. 1998;150:1–17.
-
- Erdemir D. Lee A. Y. Myerson A. S. Acc. Chem. Res. 2009;42:621–629. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

