Biosynthesis and synthetic biology of psychoactive natural products
- PMID: 33908526
- PMCID: PMC8217322
- DOI: 10.1039/d1cs00065a
Biosynthesis and synthetic biology of psychoactive natural products
Abstract
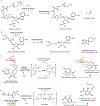
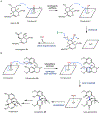
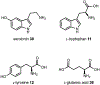
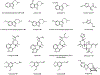
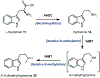
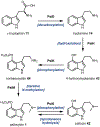
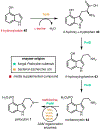
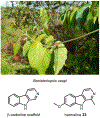
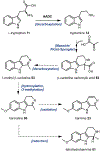
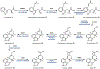
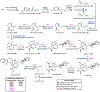
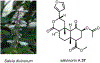
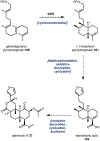
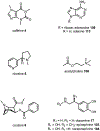
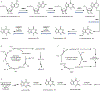
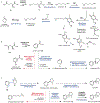
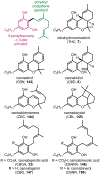
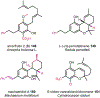
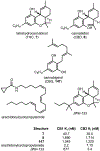
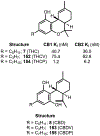
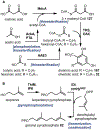
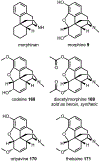
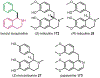
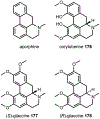
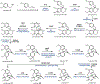
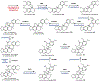
Psychoactive natural products play an integral role in the modern world. The tremendous structural complexity displayed by such molecules confers diverse biological activities of significant medicinal value and sociocultural impact. Accordingly, in the last two centuries, immense effort has been devoted towards establishing how plants, animals, and fungi synthesize complex natural products from simple metabolic precursors. The recent explosion of genomics data and molecular biology tools has enabled the identification of genes encoding proteins that catalyze individual biosynthetic steps. Once fully elucidated, the "biosynthetic pathways" are often comparable to organic syntheses in elegance and yield. Additionally, the discovery of biosynthetic enzymes provides powerful catalysts which may be repurposed for synthetic biology applications, or implemented with chemoenzymatic synthetic approaches. In this review, we discuss the progress that has been made toward biosynthetic pathway elucidation amongst four classes of psychoactive natural products: hallucinogens, stimulants, cannabinoids, and opioids. Compounds of diverse biosynthetic origin - terpene, amino acid, polyketide - are identified, and notable mechanisms of key scaffold transforming steps are highlighted. We also provide a description of subsequent applications of the biosynthetic machinery, with an emphasis placed on the synthetic biology and metabolic engineering strategies enabling heterologous production.
Conflict of interest statement
Conflicts of interest
The authors declare the following competing financial interest(s):
John Billingsley is an employee of Invizyne, Technologies (Monrovia, CA, USA), a company seeking to commercialize synthetic biochemistry.
Figures
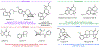












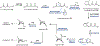
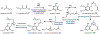

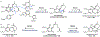

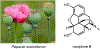
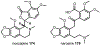
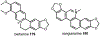

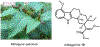
Similar articles
-
Cell-free synthetic biology for natural product biosynthesis and discovery.Chem Soc Rev. 2025 May 6;54(9):4314-4352. doi: 10.1039/d4cs01198h. Chem Soc Rev. 2025. PMID: 40104998 Free PMC article. Review.
-
Toward the Heterologous Biosynthesis of Plant Natural Products: Gene Discovery and Characterization.ACS Synth Biol. 2021 Nov 19;10(11):2784-2795. doi: 10.1021/acssynbio.1c00315. Epub 2021 Nov 10. ACS Synth Biol. 2021. PMID: 34757715 Review.
-
Synthetic Biology in Natural Product Biosynthesis.Chem Rev. 2025 Apr 9;125(7):3814-3931. doi: 10.1021/acs.chemrev.4c00567. Epub 2025 Mar 21. Chem Rev. 2025. PMID: 40116601 Review.
-
Metabolic engineering for the production of natural products.Annu Rev Chem Biomol Eng. 2011;2:211-36. doi: 10.1146/annurev-chembioeng-061010-114209. Annu Rev Chem Biomol Eng. 2011. PMID: 22432617 Free PMC article. Review.
-
A pharmaceutical model for the molecular evolution of microbial natural products.FEBS J. 2020 Apr;287(7):1429-1449. doi: 10.1111/febs.15129. Epub 2019 Nov 22. FEBS J. 2020. PMID: 31693795 Review.
Cited by
-
Tunable Nanomaterials of Intracellular Crystallization for In Situ Biolabeling and Biomedical Imaging.Chem Biomed Imaging. 2023 Mar 30;1(9):767-784. doi: 10.1021/cbmi.3c00021. eCollection 2023 Dec 25. Chem Biomed Imaging. 2023. PMID: 39473839 Free PMC article. Review.
-
Immobilized Nucleoside 2'-Deoxyribosyltransferases from Extremophiles for Nucleoside Biocatalysis.ACS Omega. 2024 Dec 30;10(1):1067-1076. doi: 10.1021/acsomega.4c08364. eCollection 2025 Jan 14. ACS Omega. 2024. PMID: 39829460 Free PMC article.
-
Unveiling nonribosomal peptide synthetases from the ergot fungus Claviceps purpurea involved in the formation of diverse ergopeptines.Acta Pharm Sin B. 2025 Jun;15(6):3321-3337. doi: 10.1016/j.apsb.2025.03.022. Epub 2025 Mar 13. Acta Pharm Sin B. 2025. PMID: 40654343 Free PMC article.
-
Polyphenolic Platform Ameliorated Sanshool for Skin Photoprotection.Adv Sci (Weinh). 2024 Apr;11(16):e2310012. doi: 10.1002/advs.202310012. Epub 2024 Feb 15. Adv Sci (Weinh). 2024. PMID: 38359060 Free PMC article.
-
Engineered Production of Strictosidine and Analogues in Yeast.ACS Synth Biol. 2022 Apr 15;11(4):1639-1649. doi: 10.1021/acssynbio.2c00037. Epub 2022 Mar 16. ACS Synth Biol. 2022. PMID: 35294193 Free PMC article.
References
-
- Merlin MD, Econ. Bot, 2003, 57, 295–323.
-
- Guerra-Doce E, Time Mind, 2015, 8, 91–112.
-
- Buenz EJ, Verpoorte R and Bauer BA, Annu. Rev. Pharmacol. Toxicol, 2018, 58, 509–530. - PubMed
-
- Efferth T, Banerjee M, Paul NW, Abdelfatah S, Arend J, Elhassan G, Hamdoun S, Hamm R, Hong C, Kadioglu O, Naß J, Ochwangi D, Ooko E, Ozenver N, Saeed MEM, Schneider M, Seo EJ, Wu CF, Yan G, Zeino M, Zhao Q, Abu-Darwish MS, Andersch K, Alexie G, Bessarab D, Bhakta-Guha D, Bolzani V, Dapat E, Donenko FV, Efferth M, Greten HJ, Gunatilaka L, Hussein AA, Karadeniz A, Khalid HE, Kuete V, Lee IS, Liu L, Midiwo J, Mora R, Nakagawa H, Ngassapa O, Noysang C, Omosa LK, Roland FH, Shahat AA, Saab A, Saeed EM, Shan L and Titinchi SJJ, Phytomedicine, 2016, 23, 166–173. - PubMed
-
- George JR, Michaels TI, Sevelius J and Williams MT, J. Psychedelic Stud, 2019, 4, 4–15.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

