Biosynthesis and synthetic biology of psychoactive natural products
- PMID: 33908526
- PMCID: PMC8217322
- DOI: 10.1039/d1cs00065a
Biosynthesis and synthetic biology of psychoactive natural products
Abstract
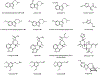
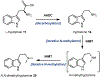
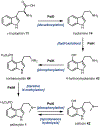
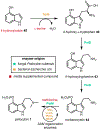
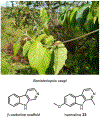
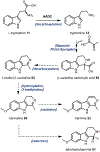
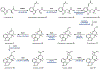
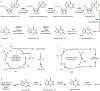
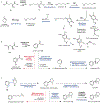
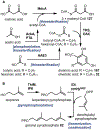
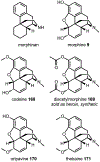
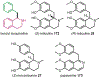
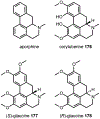
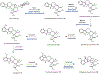
Psychoactive natural products play an integral role in the modern world. The tremendous structural complexity displayed by such molecules confers diverse biological activities of significant medicinal value and sociocultural impact. Accordingly, in the last two centuries, immense effort has been devoted towards establishing how plants, animals, and fungi synthesize complex natural products from simple metabolic precursors. The recent explosion of genomics data and molecular biology tools has enabled the identification of genes encoding proteins that catalyze individual biosynthetic steps. Once fully elucidated, the "biosynthetic pathways" are often comparable to organic syntheses in elegance and yield. Additionally, the discovery of biosynthetic enzymes provides powerful catalysts which may be repurposed for synthetic biology applications, or implemented with chemoenzymatic synthetic approaches. In this review, we discuss the progress that has been made toward biosynthetic pathway elucidation amongst four classes of psychoactive natural products: hallucinogens, stimulants, cannabinoids, and opioids. Compounds of diverse biosynthetic origin - terpene, amino acid, polyketide - are identified, and notable mechanisms of key scaffold transforming steps are highlighted. We also provide a description of subsequent applications of the biosynthetic machinery, with an emphasis placed on the synthetic biology and metabolic engineering strategies enabling heterologous production.
Conflict of interest statement
Conflicts of interest
The authors declare the following competing financial interest(s):
John Billingsley is an employee of Invizyne, Technologies (Monrovia, CA, USA), a company seeking to commercialize synthetic biochemistry.
Figures












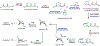
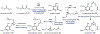

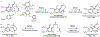

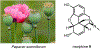
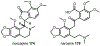

References
-
- Merlin MD, Econ. Bot, 2003, 57, 295–323.
-
- Guerra-Doce E, Time Mind, 2015, 8, 91–112.
-
- Buenz EJ, Verpoorte R and Bauer BA, Annu. Rev. Pharmacol. Toxicol, 2018, 58, 509–530. - PubMed
-
- Efferth T, Banerjee M, Paul NW, Abdelfatah S, Arend J, Elhassan G, Hamdoun S, Hamm R, Hong C, Kadioglu O, Naß J, Ochwangi D, Ooko E, Ozenver N, Saeed MEM, Schneider M, Seo EJ, Wu CF, Yan G, Zeino M, Zhao Q, Abu-Darwish MS, Andersch K, Alexie G, Bessarab D, Bhakta-Guha D, Bolzani V, Dapat E, Donenko FV, Efferth M, Greten HJ, Gunatilaka L, Hussein AA, Karadeniz A, Khalid HE, Kuete V, Lee IS, Liu L, Midiwo J, Mora R, Nakagawa H, Ngassapa O, Noysang C, Omosa LK, Roland FH, Shahat AA, Saab A, Saeed EM, Shan L and Titinchi SJJ, Phytomedicine, 2016, 23, 166–173. - PubMed
-
- George JR, Michaels TI, Sevelius J and Williams MT, J. Psychedelic Stud, 2019, 4, 4–15.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

