Evaluating four motivation-phase intervention components for use with primary care patients unwilling to quit smoking: a randomized factorial experiment
- PMID: 33908665
- PMCID: PMC8492501
- DOI: 10.1111/add.15528
Evaluating four motivation-phase intervention components for use with primary care patients unwilling to quit smoking: a randomized factorial experiment
Abstract
Aims: To assess the effectiveness of intervention components designed to increase quit attempts and promote abstinence in patients initially unwilling to quit smoking.
Design: A four-factor, randomized factorial experiment.
Setting: Sixteen primary care clinics in southern Wisconsin.
Participants: A total of 577 adults who smoke (60% women, 80% White) recruited during primary care visits who were currently willing to reduce their smoking but unwilling to try to quit. Interventions Four factors contrasted intervention components administered over a 1-year period: (i) nicotine mini-lozenge versus none; (ii) reduction counseling versus none; (iii) behavioral activation (BA) counseling versus none; and (iv) motivational 5Rs counseling versus none. Participants could request cessation treatment at any time.
Measurements: The primary outcome was 7-day point-prevalence abstinence at 52 weeks post enrollment; secondary outcomes were point-prevalence abstinence at 26 weeks and making a quit attempt by weeks 26 and 52.
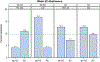
Findings: No abstinence main effects were found but a mini-lozenge × reduction counseling × BA interaction was found at 52 weeks; P = 0.03. Unpacking this interaction showed that the mini-lozenge alone produced the highest abstinence rate (16.7%); combining it with reduction counseling produced an especially low abstinence rate (4.1%). Reduction counseling decreased the likelihood of making a quit attempt by 52 weeks relative to no reduction counseling (P = 0.01).
Conclusions: Nicotine mini-lozenges may increase smoking abstinence in people initially unwilling to quit smoking, but their effectiveness declines when used with smoking reduction counseling or other behavioral interventions. Reduction counseling decreases the likelihood of making a quit attempt in people initially unwilling to quit smoking.
Trial registration: ClinicalTrials.gov NCT02354872.
Keywords: Chronic care smoking treatment; comparative effectiveness; factorial experiment; motivation phase; multiphase optimization strategy (MOST); primary care; quit attempts; smoking cessation; smoking reduction; unwilling to quit smoking.
© 2021 Society for the Study of Addiction.
Conflict of interest statement
Declaration of Interest: This research was supported by a National Cancer Institute Grant [grant P01 CA180945], National Institute on Alcohol Abuse and Alcoholism [grant R01AA022931], National Institute on Drug Abuse [grant R01DA040480] and P50DA039838], National Institute on Diabetes and Digestive and Kidney Disease [grant R01DK097364], and Department of Veterans Affairs [grant 101CX00056].
Figures


References
-
- Centers for Disease Control and Prevention. Health effects of cigarette smoking2020 20July2020. Available from: https://www.cdc.gov/tobacco/data_statistics/fact_sheets/health_effects/e....
-
- Centers for Disease Control and Prevention. Quitting smoking among adults - United States 2001–2010. MMWR 2011;60(44):1513–9. - PubMed
-
- Etter JF, Perneger TV, Ronchi A. Distributions of smokers by stage: international comparison and association with smoking prevalence. Prev Med 1997;26(4):580–5. - PubMed
-
- Wewers ME, Stillman FA, Hartman AM, Shopland DR. Distribution of daily smokers by stage of change: Current Population Survey results. Prev Med 2003;36(6):710–20. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

