Azine Activation via Silylium Catalysis
- PMID: 33908753
- PMCID: PMC8154516
- DOI: 10.1021/jacs.1c03257
Azine Activation via Silylium Catalysis
Abstract
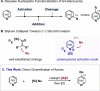
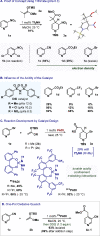
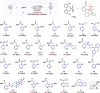
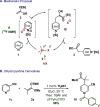
Practical, efficient, and general methods for the diversification of N-heterocycles have been a recurrent goal in chemical synthesis due to the ubiquitous influence of these motifs within bioactive frameworks. Here, we describe a direct, catalytic, and selective functionalization of azines via silylium activation. Our catalyst design enables mild conditions and a remarkable functional group tolerance in a one-pot setup.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
-
For an overview of the pharmaceutical scenery, see:
- Vitaku E.; Smith D. T.; Njardarson J. T. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U. S. FDA approved pharmaceuticals. J. Med. Chem. 2014, 57, 10257. 10.1021/jm501100b. - DOI - PubMed
- Cernak T.; Dykstra K. D.; Tyagarajan S.; Vachal P.; Krska S. W. The medicinal chemist’s toolbox for late stage functionalization of drug-like molecules. Chem. Soc. Rev. 2016, 45, 546. 10.1039/C5CS00628G. - DOI - PubMed
-
-
-
For comprehensive perspectives, see:
- Brown D. G.; Boström J. Analysis of past and present synthetic methodologies on medicinal chemistry: where have all the reactions gone?. J. Med. Chem. 2016, 59, 4443. 10.1021/acs.jmedchem.5b01409. - DOI - PubMed
- Ishihara Y.; Montero A.; Baran P. S.. The portable chemist’s consultant; Apple Publishing Group: 2016.
-
-
-
For recent reviews, see:
- Murakami K.; Yamada S.; Kaneda T.; Itami K. C−H functionalization of azines. Chem. Rev. 2017, 117, 9302. 10.1021/acs.chemrev.7b00021. - DOI - PubMed
- Zhou F.; Jiao L. Recent developments in transition-metal-free functionalization and derivatization reactions of pyridines. Synlett 2021, 32, 159. 10.1055/s-0040-1706552. - DOI
-
-
-
For representative examples, see:
- Seiple I. B.; Rodriguez R. A.; Gianatassio R.; Fujiwara Y.; Sobel A. L.; Baran P. S. Direct C–H arylation of electron-deficient heterocycles with arylboronic acids. J. Am. Chem. Soc. 2010, 132, 13194. 10.1021/ja1066459. - DOI - PMC - PubMed
- Nakao Y.; Yamada Y.; Kashihara N.; Hiyama T. Selective C4-alkylation of pyridine by nickel/Lewis acid catalysis. J. Am. Chem. Soc. 2010, 132, 13666. 10.1021/ja106514b. - DOI - PubMed
- Fier P. S.; Hartwig J. F. Selective C–H fluorination of pyridines and diazines inspired by a classic amination reaction. Science 2013, 342, 956. 10.1126/science.1243759. - DOI - PubMed
- Margrey K. A.; McManus J. B.; Bonazzi S.; Zecri F.; Nicewicz D. A. Predictive model for site-selective aryl and heteroaryl C−H functionalization via organic photoredox catalysis. J. Am. Chem. Soc. 2017, 139, 11288. 10.1021/jacs.7b06715. - DOI - PMC - PubMed
-
-
-
Selective deprotonation has also emerged to occupy a central role in the current set of methodologies when combined with electrophiles:
- Haag B.; Mosrin M.; Ila H.; Malakhov V.; Knochel P. Regio- and chemoselective metalation of arenes and heteroarenes using hindered metal amide bases. Angew. Chem., Int. Ed. 2011, 50, 9794. 10.1002/anie.201101960. - DOI - PubMed
-
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

