Common virulence gene expression in adult first-time infected malaria patients and severe cases
- PMID: 33908865
- PMCID: PMC8102065
- DOI: 10.7554/eLife.69040
Common virulence gene expression in adult first-time infected malaria patients and severe cases
Abstract
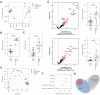
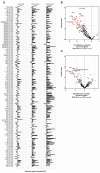
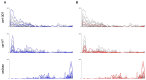
Sequestration of Plasmodium falciparum(P. falciparum)-infected erythrocytes to host endothelium through the parasite-derived P. falciparum erythrocyte membrane protein 1 (PfEMP1) adhesion proteins is central to the development of malaria pathogenesis. PfEMP1 proteins have diversified and expanded to encompass many sequence variants, conferring each parasite a similar array of human endothelial receptor-binding phenotypes. Here, we analyzed RNA-seq profiles of parasites isolated from 32 P. falciparum-infected adult travellers returning to Germany. Patients were categorized into either malaria naive (n = 15) or pre-exposed (n = 17), and into severe (n = 8) or non-severe (n = 24) cases. For differential expression analysis, PfEMP1-encoding var gene transcripts were de novo assembled from RNA-seq data and, in parallel, var-expressed sequence tags were analyzed and used to predict the encoded domain composition of the transcripts. Both approaches showed in concordance that severe malaria was associated with PfEMP1 containing the endothelial protein C receptor (EPCR)-binding CIDRα1 domain, whereas CD36-binding PfEMP1 was linked to non-severe malaria outcomes. First-time infected adults were more likely to develop severe symptoms and tended to be infected for a longer period. Thus, parasites with more pathogenic PfEMP1 variants are more common in patients with a naive immune status, and/or adverse inflammatory host responses to first infections favor the growth of EPCR-binding parasites.
Keywords: P. falciparum; PfEMP1; RNA-seq; chromosomes; gene expression; infectious disease; microbiology; transcriptomics; variant surface antigens; virulence.
© 2021, Wichers et al.
Conflict of interest statement
JW, GT, TT, RK, BK, JS, Hv, JS, HS, RW, LT, FL, AS, IB, ET, RF, TO, TL, TG, MD, AB No competing interests declared
Figures









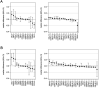

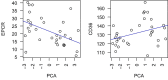
References
-
- Andrade CM, Fleckenstein H, Thomson-Luque R, Doumbo S, Lima NF, Anderson C, Hibbert J, Hopp CS, Tran TM, Li S, Niangaly M, Cisse H, Doumtabe D, Skinner J, Sturdevant D, Ricklefs S, Virtaneva K, Asghar M, Homann MV, Turner L, Martins J, Allman EL, N'Dri ME, Winkler V, Llinás M, Lavazec C, Martens C, Färnert A, Kayentao K, Ongoiba A, Lavstsen T, Osório NS, Otto TD, Recker M, Traore B, Crompton PD, Portugal S. Increased circulation time of plasmodium falciparum underlies persistent asymptomatic infection in the dry season. Nature Medicine. 2020;26:1929–1940. doi: 10.1038/s41591-020-1084-0. - DOI - PubMed
-
- Argy N, Kendjo E, Augé-Courtoi C, Cojean S, Clain J, Houzé P, Thellier M, Hubert V, Deloron P, Houzé S, CNRP study group Influence of host factors and parasite biomass on the severity of imported plasmodium falciparum malaria. PLOS ONE. 2017;12:e0175328. doi: 10.1371/journal.pone.0175328. - DOI - PMC - PubMed
-
- Avril M, Tripathi AK, Brazier AJ, Andisi C, Janes JH, Soma VL, Sullivan DJ, Bull PC, Stins MF, Smith JD. A restricted subset of var genes mediates adherence of plasmodium falciparum-infected erythrocytes to brain endothelial cells. PNAS. 2012;109:E1782–E1790. doi: 10.1073/pnas.1120534109. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

