IMI Pathologic Myopia
- PMID: 33909033
- PMCID: PMC8083114
- DOI: 10.1167/iovs.62.5.5
IMI Pathologic Myopia
Erratum in
-
Erratum in: IMI Pathologic Myopia.Invest Ophthalmol Vis Sci. 2021 Jun 1;62(7):17. doi: 10.1167/iovs.62.7.17. Invest Ophthalmol Vis Sci. 2021. PMID: 34125158 Free PMC article. No abstract available.
Abstract
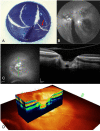
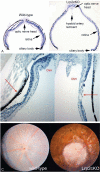
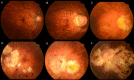
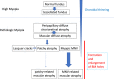
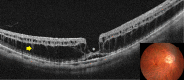
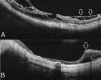
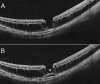
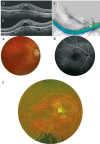
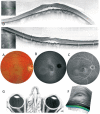
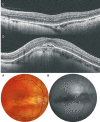
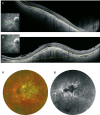
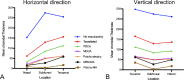
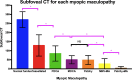
Pathologic myopia is a major cause of visual impairment worldwide. Pathologic myopia is distinctly different from high myopia. High myopia is a high degree of myopic refractive error, whereas pathologic myopia is defined by a presence of typical complications in the fundus (posterior staphyloma or myopic maculopathy equal to or more serious than diffuse choroidal atrophy). Pathologic myopia often occurs in eyes with high myopia, however its complications especially posterior staphyloma can also occur in eyes without high myopia. Owing to a recent advance in ocular imaging, an objective and accurate diagnosis of pathologic myopia has become possible. Especially, optical coherence tomography has revealed novel lesions like dome-shaped macula and myopic traction maculopathy. Wide-field optical coherence tomography has succeeded in visualizing the entire extent of large staphylomas. The effectiveness of new therapies for complications have been shown, such as anti-VEGF therapies for myopic macular neovascularization and vitreoretinal surgery for myopic traction maculopathy. Myopia, especially childhood myopia, has been increasing rapidly in the world. In parallel with an increase in myopia, the prevalence of high myopia has also been increasing. However, it remains unclear whether or not pathologic myopia will increase in parallel with an increase of myopia itself. In addition, it has remained unclear whether genes responsible for pathologic myopia are the same as those for myopia in general, or whether pathologic myopia is genetically different from other myopia.
Conflict of interest statement
Disclosure:
Figures


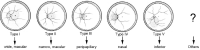


References
-
- Duke-Elder S. (ed) Pathological Refractive Errors. St. Louis: Mosby; 1970.
-
- Ohno-Matsui K, Kawasaki R, Jonas JB, et al. .. International photographic classification and grading system for myopic maculopathy. Am J Ophthalmol. 2015; 159: 877–883. - PubMed
-
- Ohno-Matsui K, Lai TY, Lai CC, Cheung CM. Updates of pathologic myopia. Prog Retin Eye Res. 2016; 52: 156–187. - PubMed
-
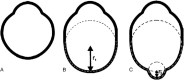
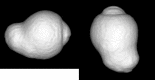
- Moriyama M, Ohno-Matsui K, Hayashi K, et al. .. Topographic analyses of shape of eyes with pathologic myopia by high-resolution three-dimensional magnetic resonance imaging. Ophthalmology. 2011; 118: 1626–1637. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

