Hydrocephalus in mouse B3glct mutants is likely caused by defects in multiple B3GLCT substrates in ependymal cells and subcommissural organ
- PMID: 33909046
- PMCID: PMC8579228
- DOI: 10.1093/glycob/cwab033
Hydrocephalus in mouse B3glct mutants is likely caused by defects in multiple B3GLCT substrates in ependymal cells and subcommissural organ
Abstract
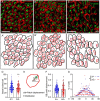
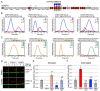
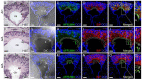
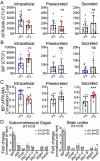
Peters plus syndrome, characterized by defects in eye and skeletal development with isolated cases of ventriculomegaly/hydrocephalus, is caused by mutations in the β3-glucosyltransferase (B3GLCT) gene. In the endoplasmic reticulum, B3GLCT adds glucose to O-linked fucose on properly folded thrombospondin type 1 repeats (TSRs). The resulting glucose-fucose disaccharide is proposed to stabilize the TSR fold and promote secretion of B3GLCT substrates, with some substrates more sensitive than others to loss of glucose. Mouse B3glct mutants develop hydrocephalus at high frequency. In this study, we demonstrated that B3glct mutant ependymal cells had fewer cilia basal bodies and altered translational polarity compared to controls. Localization of mRNA encoding A Disintegrin and Metalloproteinase with ThromboSpondin type 1 repeat 20 (ADAMTS20) and ADAMTS9 suggested that reduced function of these B3GLCT substrates contributed to ependymal cell abnormalities. In addition, we showed that multiple B3GLCT substrates (Adamts3, Adamts9 and Adamts20) are expressed by the subcommissural organ, that subcommissural organ-spondin ((SSPO) also known as SCO-spondin) TSRs were modified with O-linked glucose-fucose and that loss of B3GLCT reduced secretion of SSPO in cultured cells. In the B3glct mutant, intracellular levels of SSPO were reduced and BiP levels increased, suggesting a folding defect. Secreted SSPO colocalized with BiP, raising the possibility that abnormal extracellular assembly of SSPO into Reissner's fiber also contributed to impaired CSF flow in mutants. Combined, these studies underscore the complexity of the B3glct mutant hydrocephalus phenotype and demonstrate that impaired cerebrospinal fluid (CSF) flow likely stems from the collective effects of the mutation on multiple processes.
Keywords: C-mannosylation; O-fucosylation; SCO-spondin; hydrocephalus; thrombospondin.
© The Author(s) 2021. Published by Oxford University Press. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures


Similar articles
-
ADAMTS9 and ADAMTS20 are differentially affected by loss of B3GLCT in mouse model of Peters plus syndrome.Hum Mol Genet. 2019 Dec 15;28(24):4053-4066. doi: 10.1093/hmg/ddz225. Hum Mol Genet. 2019. PMID: 31600785 Free PMC article.
-
Peters plus syndrome mutations disrupt a noncanonical ER quality-control mechanism.Curr Biol. 2015 Feb 2;25(3):286-295. doi: 10.1016/j.cub.2014.11.049. Epub 2014 Dec 24. Curr Biol. 2015. PMID: 25544610 Free PMC article.
-
Peters plus syndrome mutations affect the function and stability of human β1,3-glucosyltransferase.J Biol Chem. 2021 Jul;297(1):100843. doi: 10.1016/j.jbc.2021.100843. Epub 2021 May 28. J Biol Chem. 2021. PMID: 34058199 Free PMC article.
-
The secretory ependymal cells of the subcommissural organ: which role in hydrocephalus?Int J Biochem Cell Biol. 2007;39(3):463-8. doi: 10.1016/j.biocel.2006.10.021. Epub 2006 Nov 2. Int J Biochem Cell Biol. 2007. PMID: 17150405 Review.
-
SCO-spondin, a glycoprotein of the subcommissural organ/Reissner's fiber complex: evidence of a potent activity on neuronal development in primary cell cultures.Microsc Res Tech. 2001 Mar 1;52(5):484-95. doi: 10.1002/1097-0029(20010301)52:5<484::AID-JEMT1034>3.0.CO;2-0. Microsc Res Tech. 2001. PMID: 11241859 Review.
Cited by
-
O-fucosylation of thrombospondin type 1 repeats is essential for ECM remodeling and signaling during bone development.Matrix Biol. 2022 Mar;107:77-96. doi: 10.1016/j.matbio.2022.02.002. Epub 2022 Feb 12. Matrix Biol. 2022. PMID: 35167946 Free PMC article.
-
O-fucosylation stabilizes the TSR3 motif in thrombospondin-1 by interacting with nearby amino acids and protecting a disulfide bond.J Biol Chem. 2022 Jun;298(6):102047. doi: 10.1016/j.jbc.2022.102047. Epub 2022 May 18. J Biol Chem. 2022. PMID: 35597280 Free PMC article.
-
Protein C-Mannosylation and C-Mannosyl Tryptophan in Chemical Biology and Medicine.Molecules. 2021 Aug 30;26(17):5258. doi: 10.3390/molecules26175258. Molecules. 2021. PMID: 34500691 Free PMC article. Review.
-
Multiciliated ependymal cells: an update on biology and pathology in the adult brain.Acta Neuropathol. 2024 Sep 10;148(1):39. doi: 10.1007/s00401-024-02784-0. Acta Neuropathol. 2024. PMID: 39254862 Review.
-
HTRA1 rs11528744, BCRA1 rs9928736, and B3GLCT rs4381465 are associated with age-related macular degeneration in a Chinese population.Front Genet. 2022 Sep 29;13:997840. doi: 10.3389/fgene.2022.997840. eCollection 2022. Front Genet. 2022. PMID: 36263425 Free PMC article.
References
-
- Amirav I, Wallmeier J, Loges NT, Menchen T, Pennekamp P, Mussaffi H, Abitbul R, Avital A, Bentur L, Dougherty GW, et al. 2016. Systematic analysis of CCNO variants in a defined population: Implications for clinical phenotype and differential diagnosis. Hum Mutat. 37:396–405. - PubMed
-
- Baas D, Meiniel A, Benadiba C, Bonnafe E, Meiniel O, Reith W, Durand B. 2006. A deficiency in RFX3 causes hydrocephalus associated with abnormal differentiation of ependymal cells. Eur J Neurosci. 24:1020–1030. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

